Lee, Kim, Chae, Kim, and Phi: Pediatric Nondysraphic Intramedullary Lipoma : Report of Two Cases and Review of the Literature
Abstract
Pediatric nondysraphic intramedullary lipoma is very rare, and only limited cases have been reported. In the present case, we present two infant patients with these pathologies who were surgically treated. Previous literature on 20 patients with these diseases who had undergone surgical treatments was analyzed. Surgical treatment should be considered in most symptomatic patients, and laminoplastic laminotomy and internal debulking of the lipoma under intraoperative neurophysiological monitoring are mostly recommended.
Key Words: Spinal cord neoplasms ┬Ę Lipoma ┬Ę Neurologic manifestations ┬Ę Laminoplasty.
INTRODUCTION
Spinal lipoma is often associated with spinal dysraphism and is located in the lumbosacral region [ 7, 8]. It is a rare lesion, accounting for less than 1% of all spinal masses [ 6, 7]. Nondysraphic intramedullary lipoma occurring in the pediatric population is even rarer, and only 20 cases have been previously reported to our knowledge [ 1- 6, 8- 10, 14, 15, 18]. Due to its rarity, optimal management of the disease has not been established [ 7, 11]. It is especially difficult to diagnose the disease and surgically remove lipomas in patients at very young ages [ 15]. Recently, we encountered two infant patients with these pathologies who were surgically treated. Based on the clinical observations of our two cases and reviews of the previous literature, we considered the clinical features, operative findings, and several precautions for surgical treatment.
CASE REPORT
Patient 1
Patient 1 was a 6-month-old female. She visited our hospital due to a developmental delay. She couldnŌĆÖt hold her head up or turn her body over. She had lower extremity weakness, especially in the right leg corresponding to motor power grade III. The motor power of the other side was grade IV. Urination and defecation were normal. On magnetic resonance imaging (MRI), there was a hyperintense mass-like lesion on T1- and T2-weighted images along the dorsal side of the spinal cord from C4 to T7 ( Fig. 1A-D). The patient was closely followed up for 2 more months, but motor development was sustained in a plateau and the rigidity of the right lower extremity increased. Due to the progression of neurological deterioration, surgical resection was performed to normalize the developmental delay and prevent further neurological deficits. C3-T7 laminotomy and durotomy were performed. The exposed pial surface was intact, implying that the mass was a true intramedullary lesion ( Fig. 1E). Midline myelotomy was performed first. The spinal cord was nearly replaced by huge lipomatous tissues. The tumor was fairly demarcated from the cord with sharp dissection at the lower portion of the mass. However, going to the upper portion of the lesion made it harder to distinguish the normal neural tissue trapped in the mass. Dissecting through a potential plane between the neural tissues and the tumor caused decreased motor evoked potentials (MEPs) of the left upper extremity, and safety margins should be secured to prevent neural injury ( Fig. 1F). Approximately 70% of the mass was removed by piecemeal resection ( Fig. 1G). Redundant duroplasty and laminoplasty were performed. The left abductor digiti minimi and abductor pollicis brevis MEPs transiently decreased to 10% of their baseline levels during the operation but recovered to 100% at the end of surgery. The right posterior tibialis somatosensory evoked potential decreased to 60% of its baseline and recovered to 90%. The tumor was later confirmed as mature adipose tissue consistent with lipoma. In the postoperative period, the patient suffered dysesthesia on both lower extremities, and the motor grade of the lower extremities deteriorated to I-II. She underwent intensive rehabilitation. After 1 year and 10 months, the size of the lipoma was slightly increased on follow-up MRI. However, the motor power of the lower extremities improved to motor grade IV, bilaterally. She could walk alone with a walker, and the rigidity of both lower extremities decreased. She could also grab and pinch well with both hands.
Patient 2
Patient 2 was a 2-month-old female. It was impossible to hold her due to severe back and leg pain. She also presented with quadriparesis. The motor power of both upper extremities were grade I, and those of the lower extremities were grade III. On MRI, there were multiple intramedullary mass lesions. Three isolated masses were located at the C3-T5, T6-T10, and L2-L3 levels. They showed high signal intensity on T1- and T2-weighted images ( Fig. 2A-D). Electromyography (EMG) and a nerve conduction study (NCS) showed bilateral C5-8 myeloradiculopathy with partial axonal involvement. C3-T10 laminotomy was performed, and the masses were removed under microscopic view using an ultrasonic aspirator. The lesions were firmly attached to the cord and were intermingled with normal neural tissue and nerve roots. The upper (C3-T5) and middle (T6-T10) masses were separated by a segment of normal neural tissue ( Fig. 2A, E, F). Approximately 50-60% of the mass was partially removed in the range of maximal safe margin ( Fig. 2G). The lesion located at the conus was not removed because it seemed to have no mass effect on the cord on MRI and EMG/NCS. Redundant duroplasty and laminoplasty were performed. The MEP of the right upper extremity transiently decreased to 10% of its baseline level but recovered to 100% at the end of surgery. The mass was histologically confirmed as a lipoma. Follow-up MRI after 5 months showed a slightly increased size of the lipoma at the C-T levels and no change in the lipoma at the conus compared with immediate postoperative MRI. However, with intensive rehabilitation, the motor power of the upper extremities gradually improved to grade III, and the gross motor function measure of the lower extremities was grade V. She could raise both arms, grasp, and kick. The severe back and leg pain disappeared.
Review of previous reports on pediatric intramedullary lipoma
Twelve previous reports describing twenty patients with pediatric intramedullary lipoma were found [ 1- 6, 8- 10, 14, 15, 18] ( Table 1). All 20 patients had undergone surgical treatments. In total, 22 patients including our two patients were analyzed. Ages ranged from 2 months to 19 years. There were 10 males and 12 females. Clinical presentations were neck pain, back pain, trunk or lower extremity dysesthesia, quadri- or paraparesis, and urinary difficulty. Motor weakness presented as a developmental delay at very young ages. Duration of symptoms ranged from 2 weeks to 15 years. Symptoms like pain and dysesthesia were usually insidious and chronic. Symptoms of motor deficit seemed more progressive. In some older patients, symptoms of pain and dysesthesia donŌĆÖt develop for long periods [ 10]. The lesions were mostly located at the C-T level (21 of 22 patients). Only five of 22 patients had lumbar lesions [ 2, 8, 14, 15], seven patients had intracranial extension [ 2- 6, 8, 14], and three patients showed multiple lesions [ 8, 14]. Most patients underwent operations due to neurological deterioration. Only two patients underwent surgery without neurological problems [ 6, 8]. Lipoma was partially resected in most cases because of the poor dissection plane. In only one case, a lipoma was totally removed. In this report, it was possible to grossly remove all lipomatous tissues because they were easily removed by an ultrasonic aspirator. The authors said it was an exceptional case, and partial resection was usually recommended in most lipoma cases [ 9]. The symptoms deteriorated only in two cases after the operation [ 6]. In some cases, preoperative symptoms deteriorated in the immediate postoperative period. However, after a longer period, symptoms gradually improved in most cases. In case 3 reported by Kim et al. [ 8] the lower extremity weakness of the patient was aggravated transiently after the operation (American Spinal Cord Injury Association [ASIA] impairment scale; D to C). It improved to the preoperative state after 4 months. In other reports [ 9, 18], weakness of the lower extremities improved after at least 1 month had passed. A delayed spinal deformity developed in one patient with extensive C6-L5 lesions [ 15]. Lipoma regrowth was identified, and repeated surgery was performed in four patients [ 6, 10]. The follow-up period ranged from the immediate postoperative period to 12 years. Our second patient is a very unique case in that she is the youngest patient and there were three isolated lesions, including a rare lumbar lesion that had never been reported.
DISCUSSION
Pathogenesis
Spinal lipoma is usually considered a congenital lesion. It is mostly believed to develop from embryological errors [ 2, 6, 7, 12, 13, 16]. Although they have the same pathological features, there are some differences between nondysraphic intramedullary lipomas and dysraphic lipomas. Dysraphic lipomas are usually detected in pediatric patients, whereas nondysraphic lipomas are mostly detected in the 2nd and 3rd decades of life [ 9]. It is assumed that dysraphic features can be more easily recognized without symptoms and neurological symptoms usually develop at younger ages in dysraphic lipomas. Furthermore, dysraphic lipomas are usually located in the lumbosacral region, but nondysraphic lipomas are mostly located in the cervicothoracic region [ 2]. They may occur from the same origin, but there may be subtle differences in the degree and time of developmental error. Although the exact pathogenesis of nondysratphic lipomas is still unclear, it has been suggested that the embryologic mechanism of nondysraphic lipomas is similar to that of dysraphic lipomatous malformations [ 2, 6- 8]. In the early developmental stage, there can be errors in the migration and differentiation of mesenchyme, especially when forming the neural tube [ 2, 6, 7, 12, 13, 16]. By the end of the 3rd week of development, the neural tube forms from the neural plate in a process known as primary neurulation. The cutaneous ectoderm separates from the neuroectoderm, which is referred to as the process of ŌĆ£dysjunctionŌĆØ, after fusion of the neural tube [ 12, 13, 16]. Subtle errors in this process regulated by molecular interactions of signaling molecules and transcription factors can result in the mesenchyme migrating into the developing neural tube. It is postulated that ŌĆ£premature dysjunctionŌĆØ in the surface ectoderm allows invasion of the surrounding mesodermal tissues into the dorsal surface of the incompletely closed neural tube. Incarcerated mesodermal tissues gain access to the ependymal surface of the developing neural tube and can differentiate into fat, evolving into a spinal lipoma [ 2, 6, 7]. It is, therefore, a hamartoma or malformation rather than a true neoplasm [ 2]. A more exact pathogenesis of the disease should be speculated.
Treatment strategies
A nondysraphic intramedullary lipoma in childhood is extremely rare. Because its clinical course is usually indolent with nonspecific symptoms and mild symptoms are hard to recognize at young ages, detection of the disease can be late. Furthermore, symptoms can progress rapidly after initial presentation, so the disease can be detected when patients are in a poor neurological state. The symptoms often improve in the long term when patients receive appropriate treatment in time. However, severe neurological deficits often partially recover or do not improve, so early detection and timely treatment are important.
Little is known about the proper timing of surgical treatments in nondysraphic intramedullary lipoma [ 6]. By reviewing 20 patients in the literature and our two patients, there were differences in the duration of symptoms among patients. For our two patients, initial neurological deficits were definite and symptoms had progressed. But surgery was delayed as much as possible because they were too young. On the other hand, patient 1 reported by Lee et al. [ 10] had suffered long-standing back and leg pain for 15 years and underwent surgical treatment due to recent aggravation of paraparesis with gait disturbance for 3 months. There seemed possibility of a correlation between age and symptom duration. Age can be a significant factor in affecting the progression of symptoms and determining the timing of surgical treatment. In the case of ŌĆ£dysraphicŌĆØ lipoma, pediatric patients usually show rapid progression of symptoms. On the contrary, adult patients with ŌĆ£dysraphicŌĆØ lipoma often show no symptoms or non-progressive mild symptoms, and they can be managed without the operation [ 17]. This speculation of the relationship between age and symptomatic changes seems similar in nondysraphic and dysraphic lipoma. As in dysraphic lipoma, nondysraphic lipoma patients with no symptoms or non-progressive mild symptoms can be followed-up without surgical treatment. We do not advocate preventive surgery because symptoms donŌĆÖt progress for a long time in some patients. Also, surgical treatment carries significant risks of neurological deterioration and spinal deformity. Patients with progressive neurological symptoms should be considered for surgical treatments. The most commonly performed operative procedures are laminoplastic laminotomy and partial decompression of the lipoma. Gross total resection should not be tried in most cases, because there is no clear dissection plane between the lipoma and normal neural tissue and part of the lipoma is intermingled with neural tissue. The purpose of the surgery should be to prevent further neurological deterioration and expect maximal recovery with intensive rehabilitation. Additionally, lipomas can be easily diagnosed before surgery using MRI, and it can be assumed that there is no malignancy. However, internal debulking of the lesion rather than simple bony decompression should be considered because the lipoma can grow after the operation. Intraoperative monitoring (IOM), ultrasonic aspirator, or CO2 laser should be utilized to remove the maximal lipoma within the safe margin. Redundant duroplasty can be performed to provide capacity to the cord, and laminoplasty should be performed because deformity can occur during growth.
After the operation, all patients should be followed up for regrowth of the lipoma. In a previous case reported by Yilmaz and Aydemir [ 18], the size of the lipoma decreased over time after the operation. However, it was an extremely rare event. In most cases, residual lipoma showed minimal change in size or regrew over time. A small number of patients needed reoperation (four of 20 patients [ 6, 10] ), but other patients might need another surgery repeated if followed up for a longer period. The patients should be followed up for neurological deterioration and spinal deformities.
CONCLUSION
Pediatric nondysraphic intramedullary lipoma is extremely rare. Because disease progression can cause permanent neurological impairment, early detection of the disease and timely treatment of symptomatic patients are important. Younger patients can show the early progression of symptoms and surgical treatment should be considered in all patients with neurological deterioration. Laminoplastic laminotomy and internal debulking of the lipoma in the range of safe margins under IOM are recommended. All patients should be followed up for the emergence of lipoma regrowth, neurological changes, and spinal deformities.
Acknowledgements
This work was supported by Creative-Pioneering Researchers Program through Seoul National University (800- 20210571; to Phi JH).
Fig.┬Ā1.
Magnetic resonance imaging (MRI) and intraoperative images of patient 1. Preoperative T1-weighted MRI (A). Sagittal image with corresponding axial images (B-D). A homogenous and hyperintense intramedullary mass is shown along the dorsal side of the spinal cord from C4 to T7. E and F : Intraoperative images of the upper pole of the lipoma. E : The thick pial membrane is exposed after the durotomy. The pial surface is intact, implicating that the lesion is a true intramedullary lipoma. F : The lipoma is cautiously pulled with forceps and removed to find the surface of the normal cord. The right side of the lipoma is the surface of the cord partially covered with blood. The lesion is fairly demarcated from the cord with sharp dissection at the lower portion of the lipoma. However, going to the upper portion of the lesion makes it harder to distinguish normal neural tissue trapped in the lipoma. The lipoma is intermingled with neural tissue. When dissecting through a potential plane between the neural tissues and the lipoma at this level (B), the motor evoked potential of the left upper extremity decreases. The operation is ceased to prevent further neural injury. G : Postoperative T1- weighted sagittal MRI. Approximately 70% of the lipoma is removed. 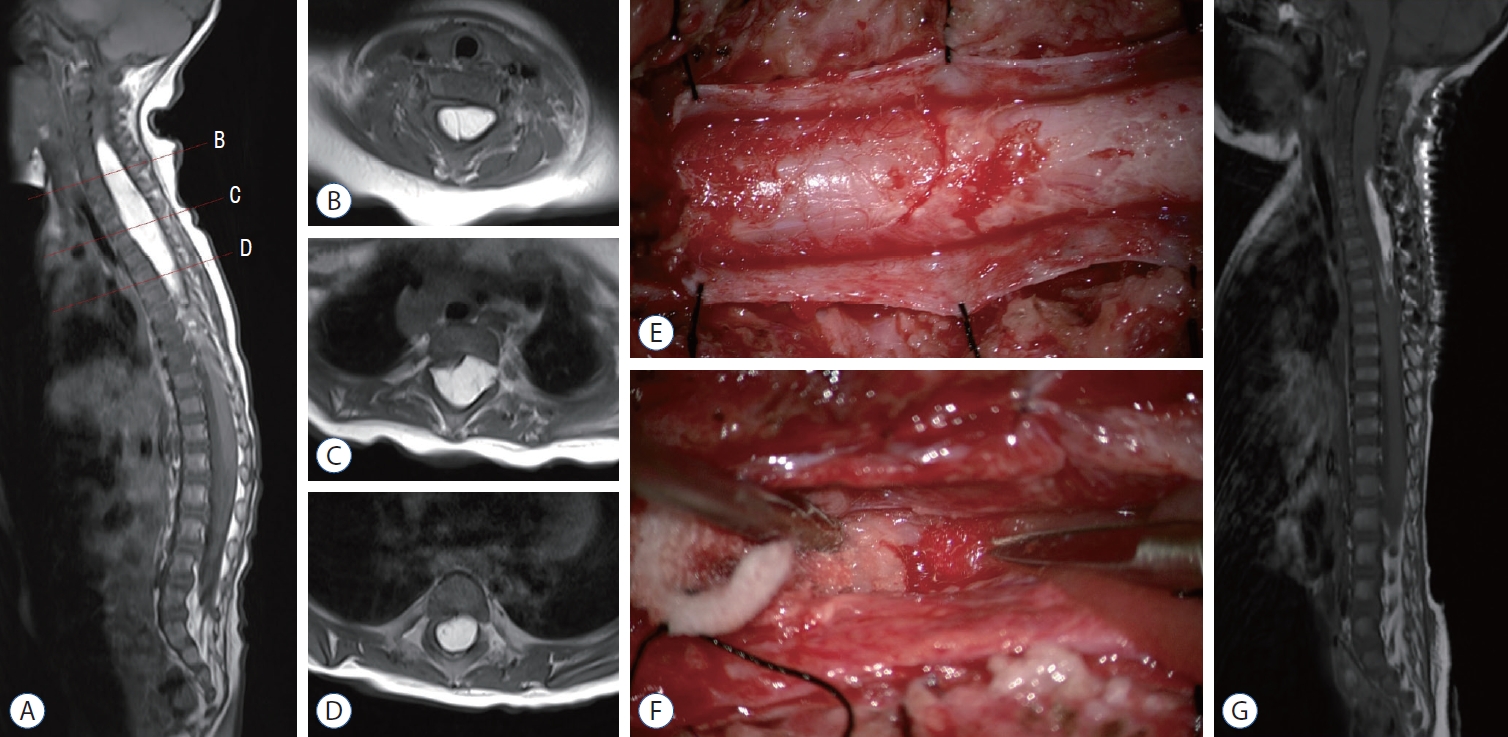
Fig.┬Ā2.
Magnetic resonance imaging (MRI) and intraoperative images of patient 2. Preoperative T1-weighted MRI (A). Sagittal image with corresponding axial images (B-D). Three isolated masses are located at the C3-T5, T6-T10, and L2-L3 levels. Homogenous and hyperintense intramedullary masses are shown along the dorsal side of the spinal cord. The upper (C3-T5) and middle (T6-T10) masses are separated by a segment of normal neural tissue (arrows in A, E, and F). E and F : Intraoperative images of the lipoma located at C3-T5 and T6-T10. E : Two isolated masses bulge out after the durotomy. There is a transitional zone (arrow) between the two masses that appears to be the normal cord covered by engorged veins. F : The masses are cautiously removed using an ultrasonic aspirator. The operation ceases when the motor evoked potential of the left upper extremity decreases. The transitional zone (arrow) is secured. G : Postoperative T1-weighted sagittal MRI. Approximately 50-60% of the lipoma is removed. 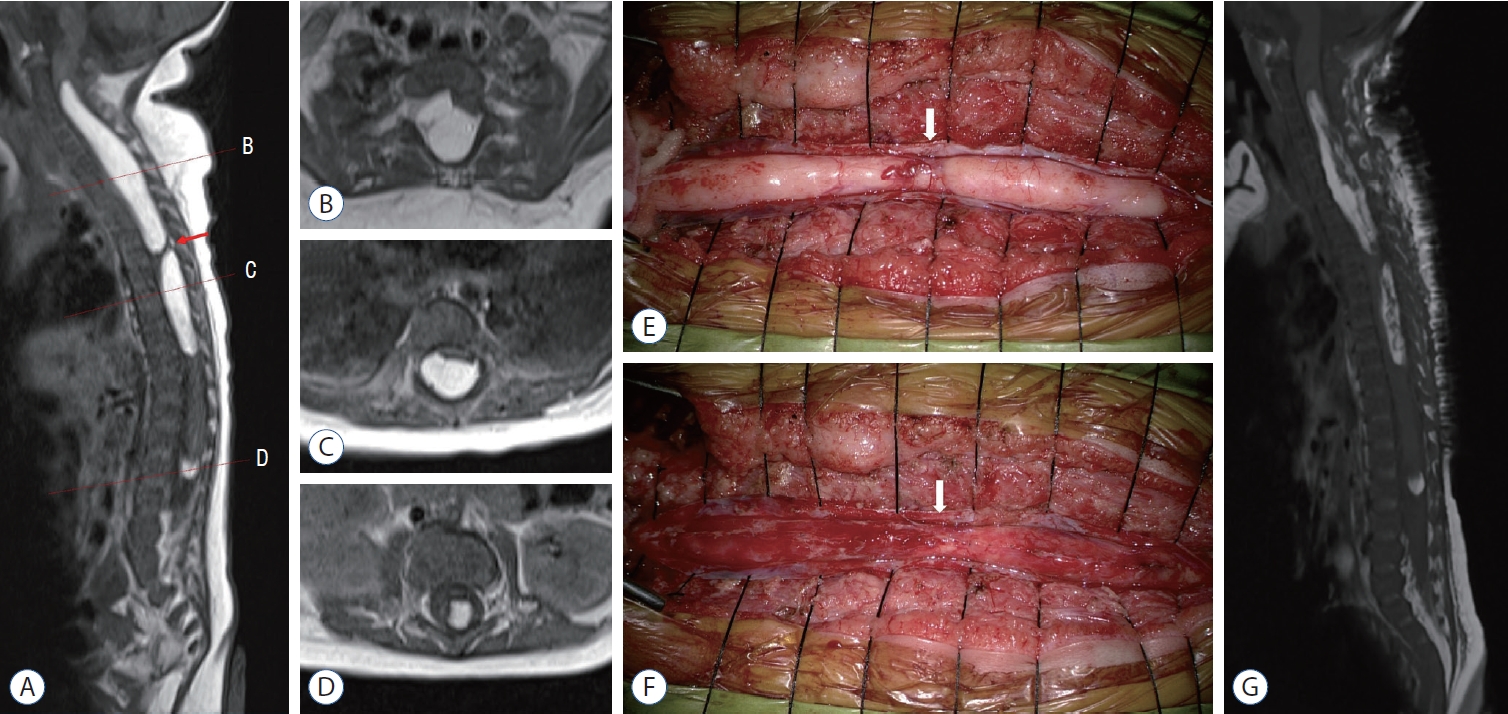
Table┬Ā1.
Summary of 20 pediatric patients with intramedullary lipoma in the literature and 2 patients in the present study
|
Study |
Age/sex |
Level of lesion |
Preoperative symptoms |
Duration of symptoms*
|
Operation |
Amount of tumor removed |
Postoperative change of symptoms |
Recurrence (repeated surgery) |
Follow-up period |
|
Lee et al. [10] (1995) |
8 years/M |
C1-7 |
Back pain, dysesthesia, quadriparesis, GD |
6 years (3 months) |
Yes |
40% |
Improved, normal gait |
Yes |
2 years 6 months |
|
Lee et al. [10] (1995) |
19 years/F |
C7-T4 |
Back pain, dysesthesia, paraparesis, GD |
15 years (3 months) |
Yes |
60% |
No change |
Yes |
7 years |
|
Chaskis et al. [5] (1997) |
3 months/M |
ICE, C1-7 |
Quadriparesis |
1 month |
Yes |
STR |
No change |
No |
1 year |
|
Kim et al. [8] (2003) |
6 months/F |
ICE, C1-T1 |
Lt. hemiparesis |
4 months |
Yes |
STR |
Improved |
No |
8 years |
|
Kim et al. [8] (2003) |
7 months/M |
T9-12 |
Lower back mass |
NA |
Yes |
STR |
No change |
No |
NA |
|
No neurological deficit |
|
Kim et al. [8] (2003) |
12 years/M |
C6-11 & L1-2 |
Lt. hemiparesis |
NA |
Yes (both lesions) |
STR |
No change |
No |
4 months |
|
Bhatoe et al. [2] (2005) |
6 years/M |
ICE, C1-4 |
Dysesthesia |
NA |
Yes |
60-80% |
No change |
No |
2 years |
|
Motor gr. III/V |
|
Bhatoe et al. [2] (2005) |
12 years/F |
L1-2 |
Dysesthesia |
NA |
Yes |
60-80% |
Improved, relief of dysesthesia |
No |
2 years |
|
Motor gr. IV/V |
|
Chagla et al. [4] (2008) |
17 years/M |
ICE, C1-6 |
Spastic quadriparesis, sensory ataxia, GD |
1 month |
Yes |
STR |
Improved, no weakness |
No |
1 year 6 months |
|
Muthusubramanian et al. [14] (2008) |
16 years/F |
ICE, C1-6 & conus |
Neck & lower back pain, foot-grip weakness, spasticity |
3 months |
Yes (both lesions) |
STR |
Improved, walk independently, relief of spasticity |
No |
NA |
|
Fleming et al. [6] (2010) |
2 months/M |
C2-T8 |
Back pain |
NA |
Yes |
50-60% |
Improved, no pain, motor gr. IV+/V |
No |
4 years |
|
Rt. hemiparesis |
|
Fleming et al. [6] (2010) |
13 months/F |
ICE, C1-7 |
No pain |
NA |
Yes |
50% |
Deteriorated, Rt. UEx. pain |
Yes |
8 years |
|
Fleming et al. [6] (2010) |
33 months/F |
C7-T7 |
Neck pain |
NA |
Yes |
10% |
Deteriorated, neck pain, motor gr. III/V |
Yes |
11 years |
|
Motor gr. IV+/V |
|
Fleming et al. [6] (2010) |
35 months/F |
C5-T8 |
Back pain |
NA |
Yes |
60-70% |
Improved, no pain, motor gr. IV+/V |
No |
12 years |
|
Rt. hemiparesis |
|
Fleming et al. [6] (2010) |
5 years/M |
C7-T6 |
Motor gr. IV-/V |
NA |
Yes |
60-70% |
Improved, motor gr. V/V |
No |
1 year |
|
Kumar et al. [9] (2011) |
14 months/M |
C7-T12 |
Developmental delay |
4 months |
Yes |
GTR |
Improved, motor gr. V/V |
No |
6 months |
|
Motor gr. III/V |
|
Nguyen and Lew [15] (2017) |
2 months/M |
C6-L5 |
Rt. leg monoplegia |
NA |
Yes |
STR |
Improved, Rt. leg motor gr. II, progression of kyphosis and scoliosis |
No |
2 years 6 months |
|
Yilmaz and Aydemir [18] (2018) |
3 years/F |
T8-9 |
Back pain, unable to walk independently, urinary incontinence |
6 months |
Yes |
50% |
Improved, walk independently |
No |
2 years |
|
Bishnoi et al. [3] (2020) |
14 months/F |
Pons-T2 |
Lt. UEx. monoplegia, swallowing difficulty |
6 months |
Yes |
STR |
Improved, Lt. UEx. motor gr. III |
No |
6 months |
|
Abuzayed et al. [1] (2021) |
14 months/F |
T1-6 |
Back pain, dysesthesia Motor gr. I/0 |
5 months (1 week) |
Yes |
STR |
Improved, motor gr. II/I |
No |
Early postop. period |
|
Current cases |
6 months/F |
C4-T7 |
Developmental delay |
2 months |
Yes |
70% |
Improved, assisted walk |
No |
1 year 10 months |
|
Motor gr. III/IV |
Motor gr. IV/IV |
|
Current cases |
2 months/F |
C3-T5, T6-10, L2-3 |
Leg pain, quadriparesis |
2 weeks |
Yes (C3-T5, T6-10 lesions) |
50-60% |
Improved, no pain |
No |
4 months |
|
UEx. motor gr. I, LEx. motor gr. III |
UEx. motor gr. III, LEx. motor gr. IV-V |
References
1. Abuzayed B, Alawneh K, Al Qawasmeh M, Raffee L : Nondysraphic spinal intramedullary lipoma: a rare case and management. Turk Arch Pediatr 56 : 85-87, 2021    2. Bhatoe HS, Singh P, Chaturvedi A, Sahai K, Dutta V, Sahoo PK : Nondysraphic intramedullary spinal cord lipomas: a review. Neurosurg Focus 18 : ECP1, 2005  3. Bishnoi I, Singh P, Duggal G, Sorout S : A rare case of intramedullary lipoma of brainstem to thoracic cord--what to do? J Pediatr Neurosci 15 : 145-149, 2020    4. Chagla AS, Balasubramaniam S, Goel AH : A massive cervicomedullary intramedullary spinal cord lipoma. J Clin Neurosci 15 : 817-820, 2008   5. Chaskis C, Michotte A, Geffray F, Vangeneugden J, Desprechins B, DŌĆÖHaens J : Cervical intramedullary lipoma with intracranial extension in an infant. Case illustration. J Neurosurg 87 : 472, 1997  6. Fleming KL, Davidson L, Gonzalez-Gomez I, McComb JG : Nondysraphic pediatric intramedullary spinal cord lipomas: report of 5 cases. J Neurosurg Pediatr 5 : 172-178, 2010  7. Kabir SM, Thompson D, Rezajooi K, Casey AT : Non-dysraphic intradural spinal cord lipoma: case series, literature review and guidelines for management. Acta Neurochir (Wien) 152 : 1139-1144, 2010    8. Kim CH, Wang KC, Kim SK, Chung YN, Choi YL, Chi JG, et al : Spinal intramedullary lipoma: report of three cases. Spinal Cord 41 : 310-315, 2003    9. Kumar A, Chandra PS, Bisht A, Garg A, Mahapatra AK, Sharma MC : Successful surgical excision of a nondysraphic holodorsal intramedullary lipoma in a 14-month-old child. Pediatr Neurosurg 47 : 272-274, 2011    10. Lee M, Rezai AR, Abbott R, Coelho DH, Epstein FJ : Intramedullary spinal cord lipomas. J Neurosurg 82 : 394-400, 1995   11. Misawa H, Oda Y, Yamane K, Tetsunaga T, Ozaki T : Maximal resection of intramedullary lipoma using intraoperative ultrasonography: a technical note. Acta Med Okayama 75 : 239-242, 2021  12. Morioka T, Murakami N, Shimogawa T, Mukae N, Hashiguchi K, Suzuki SO, et al : Neurosurgical management and pathology of lumbosacral lipomas with tethered cord. Neuropathology 37 : 385-392, 2017    13. Morota N, Ihara S, Ogiwara H : New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr 19 : 428-439, 2017   14. Muthusubramanian V, Pande A, Vasudevan MC, Ramamurthi R : Concomitant cervical and lumbar intradural intramedullary lipoma. Surg Neurol 69 : 314-317, 2008   15. Nguyen HS, Lew S : Extensive multilevel split laminotomy for debulking a cervicothoracolumbar nondysraphic intramedullary spinal-cord lipoma in a 2-month-old infant. Pediatr Neurosurg 52 : 189-194, 2017    16. Sarris CE, Tomei KL, Carmel PW, Gandhi CD : Lipomyelomeningocele: pathology, treatment, and outcomes. Neurosurg Focus 33 : E3, 2012   17. Wykes V, Desai D, Thompson DN : Asymptomatic lumbosacral lipomas- -a natural history study. Childs Nerv Syst 28 : 1731-1739, 2012    18. Yilmaz C, Aydemir F : Thoracic intramedullary lipoma in a 3-year-old child: spontaneous decrease in the size following incomplete resection. Asian J Neurosurg 13 : 188-190, 2018   
|
|


















