Rahman, Venkataram, Habib, Jahan, Raihan, Alam, Mahmood, Umana, and Chaurasia: Synchronous Carotid Body and Glomus Jugulare Tumors : A Case Report and Review of Literature
Abstract
Paragangliomas are rare neuroendocrine tumors that are usually benign in nature. They may be either familial or sporadic in their occurrence. Numerous neuroendocrine tumors are collectively included under the umbrella of paragangliomas. Among them, carotid body tumors and glomus jugulare tumors are extremely rare. Thus, we present a rare case of 29-year-old male who was admitted with hearing difficulties and tinnitus in the left ear, with swelling on the left side of the neck. Based on clinical and radiological findings, a diagnosis of left-sided glomus jugulare with carotid body tumor was made. The patient underwent a twostage surgery with an interval of approximately 2 months. Histopathology revealed a paraganglioma. Herein, we present the clinical features, imaging findings, management, and a brief review of literature on the classification, evaluation, and management of carotid body and glomus jugulare tumors. Paraganglioma is a slow-growing tumor. The synchronous occurrence of carotid body and glomus jugulare tumors is infrequent. Microsurgical resection remains the primary treatment modality. Therefore, our patient underwent two-stage surgery. The rarity of occurrence and the proximity and adherence to vital neurovascular structures have resulted in the treatment of paragangliomas remaining a challenge.
Key Words: Paraganglioma ┬Ę Carotid body tumor ┬Ę Glomus jugulare tumor.
INTRODUCTION
Carotid body tumor and glomus jugulare tumor are highly vascular neuroendocrine tumors which are classified as paraganglioma. These are usually slow growing benign tumors. Although benign in nature they sometimes show malignant potential. Paraganglioma is a very rare tumour and concurrent carotid body with glomus jugulare tumor is extremely rare. Previously only two cases [ 23, 27] of concurrent carotid body and glomus jugulare tumor have been documented. We are reporting a case of 29-year-old male who was suffering from concurrent carotid body tumor and glomus jugulare tumor on the left side, along with his management and literature review.
CASE REPORT
A 29-year-old normotensive and nondiabetic male was admitted with the complaints of difficulty in hearing for 4 years, tinnitus for 3 years in left ear. He also complained about a swelling in the left side of the neck which was gradually progressive for last 3 years. In addition to this he also mentioned about occasional headache, vertigo and respiratory distress for last 3 years. On examination there was a palpable mass present on the left upper part of the neck below and posterior to the angle of the mandible. The mass was painless, nontender, ill-defined and firm in consistency. There was no palpable pulsation or audible bruit. He did not give any positive family history.
His ultrasonography of the neck revealed an elongated slightly irregular hypoechoic solid mass about 3.8├Ś1.74 cm in the left lateral part of the neck separated from the left thyroid gland. The contrast computed tomography (CT) scan of neck and brain revealed a 3.6├Ś2.4 cm contrast enhancing lesion on the left upper part of the neck which was isodense in non-contrast CT ( Fig. 1A and B). The lesion had both inracranial and extracranial extension ( Fig. 1C and D). The central part of the lesion was inhomogeneously enhanced. The lesion was splayed between internal carotid artery (ICA) and external carotid artery (ECA), ECA was completely engulfed, ICA was adherent to the tumor and the anterior wall of the left internal jugular vein (IJV) was abutted by the lesion. Left IJV was also slightly smaller in diameter in comparison to right side. There was another lesion near the left jugular foramen with both intra and extracranial extension. Magnetic resonance imaging (MRI) of the brain ( Fig. 2) revealed an iso to hyperintense lesion present in T1W image in the left jugular foramen with multiple hypointense area within the lesion which was isointense in T2W image. The lesion was enhanced heterogeneously on gadolinium contrast (3 antero-posterior├Ś2 right left├Ś3.3 cm). CT angiogram of the neck vessel ( Fig. 3A and B) clearly showed an elongated mass which originated from the left carotid bifurcation and travelled upward in between the ECA and ICA splaying the ECA and ICA. Left ECA was engulfed by the lesion. MR venogram ( Fig. 3C and D) demonstrated reduction of venus drainage in the left transverse-sigmoid junction. Left IJV was not very well visualized. But right sigmoid sinus and IJV were patent. Our clinico-radiological diagnosis was a case of left carotid body tumor Shamblin type 3 and left glomus jugulare tumor Fish class D. We planned for two stage surgery with first removal of carotid body tumor then removal of glomus jugulare tumor in two separate occasion. For removal of carotid body tumor a slightly curved incision was made behind the posterior border of left sternocleidomastoid muscle ( Fig. 4A) which was extended about 2 cm above the left medial head of the clavicle to 1 cm below the tip of left mastoid process. Common facial vein was ligated, cut and IJV mobilized laterally. The tumor was about 5├Ś2├Ś2.5 cm. There was well developed plane between the tumor and the ICA but ECA was engulfed and it was sacrificed ( Fig. 4B- D). During the dissection left common carotid artery was injured and it was repaired with 6-0 prolene. Sudden vasospasm also developed which was managed with instillation of papaverine both intra-arterial and over the vessel. His postoperative period after 1st surgery was uneventful. Macroscopically the tumor was nodular microscopic examination sections of the nodule showed a neoplasm, composed of polygonal and oval cells arranged in nests with surrounding vascular stroma which is suggestive of carotid body tumor.
Second surgery was done 54 days later. This time a C shaped incision was made centering the left pina about 2.5 cm behind the ear ( Fig. 5). Then dissection was performed on the craniobasal area and craniectomy was done down to jugular foramen through transcondylar area. The tumor was separated from the jugular first below and after cutting the dura from above. A lower cranial nerve was seen within the tumor which was sacrificed. Then base of the skull was reconstructed using fat, fascia lata, surgicel and gelfoam. After second surgery patient developed partial VII, IX-XII cranial nerve palsy on the left side. Patient was normotensive before the 1st surgery and there was no significant fluctuation of his blood pressure occurred during and after the surgery in both occasions. Although hybrid approach for the management of this kind of tumor can be done but it was not possible for poor financial condition of the patient.
DISCUSSION
Definition
Carotid body tumor and glomus jugulare tumor are highly vascular neuroendocrine tumor which were collectively previously called chemodectoma (now obsolete). Now they are called paraganglioma [ 10]. There usually are located in the jugular fossa, carotid bifurcation, vagus nerve and other parts of the body. They arise from specialized neural crest cells associated with autonomic ganglia [ 4]. So far to our knowledge only seven cases with concurrent carotid body and glomus jugulare tumor was reported [ 3, 5, 6, 9, 14, 22, 24].
History
During development certain neural crest cells become glands instead of neurons and are called chromaffin bodies or paraganglia (largest gland is called adrenal medulla). Hence glomus jugulare, glomus tympanicum tumors are previously called intracranial pheochromocytoma. They have been also called chromaffinoma due to chromium salt affinity and chemodectoma (previously thought they all contain chemoreceptor cells) but now they are called paraganglioma because of their anatomical location [ 21].
Demography
Paraganglioma is rare with incidence of 1/300000 [ 18]. The incidence of carotid body tumor is 0.07/100000. The incidence of glomus jugulare is 1/1300000 [ 26]. Both carotid body and glomus jugulare has a female preponderance with male : female 1 : 6. Common age of onset is 50-60 years [ 8].
Common site
Paraganglioma in the central nervous system (CNS) are rare. They primarily occur in the cauda equina/filum terminale region. Outside the CNS they are designated by a name which confers the site of origin shown in Table 1. Paraganglioma may be found at other sites, including the periaortic area, trachea, larynx, mandible, nose, ciliary ganglion and fallopian cana [l7,20].
Pathophysiology
They are usually benign, hyper vascular, slow growing tumor (<2 cm/5 years) but rapidly growing tumor may also occurs. Only 1% to 5% of them are malignant [ 2]. Paragangliomas can secrete catecholamines and serotonin regardless of their location, which can result in episodic arrhythmias, hypertension, diaphoresis, headaches, and potential hypertensive crises. Any manipulation of the tumor, such as with surgery, angiography, embolization, or even palpation of the mass, could release these vasoactive substances and precipitate a hypertensive crisis. For this reason, patients suspected of having an actively secreting tumor should be screened preoperatively by testing their urine for vanillylmandelic acid and 5-hydroxyindoleacetic acid [ 18]. Paraganglioma may occur in two patterns : 1) familial : non multicentric up-to 50% and 2) nonfamilial : multicentric 5% [ 26]. These tumors usually possess secretory granules (even the functionally inactive tumors) and may actively secrete catecholamines (similar to pheochromocytomas, occur in only 1-4% of gastro jejunal tumor) [ 12, 13].
Carotid body tumors
Possibly most common is paraganglioma (some study mentioned Pheochromocytoma is more common). Five percent cases may be bilateral but incidence increases up to 26% in familial cases (autosomal dominant). Carotid body tumors consist of epithelioid cells grouped into cords or clusters, also known as Zellballen. Typically, the cells are polyhedral with granular cytoplasm. Vascular endothelial growth factor and platelet-derived endothelial cell growth factor are expressed in carotid body tumors and may contribute to the extreme vascularity of these tumors [ 1].
Glomus jugulare tumor
They are very vascular. Main feeders are from the ECA (especially inferior tympanic branch of ascending pharyngeal artery, and branches of posterior auricular, occipital, and internal maxillary), with additional feeders from petrous portion of the ICA.
Clinical features
Clinical features of paraganglioma can be divided in two categories : 1) due to release of chemical mediators : these features developed due to release of catecholamine, serotonin and kallikrein which was mentioned before and 2) due to specific location of the paraganglioma.
Clinical features of glomus jugulare
Patients commonly present with hearing loss and possible tinnitus, dizziness and ear pain may also occur. hearing loss may be conductive due to obstruction of the ear canal or sensory neural due to infection of the labyrinth. Various combination of lower cranial nerve palsy may occur. Ataxia and or hydrocephalus can occur.
Clinical features of carotid body tumor
Usually it is painless slow growing mass present in the upper part of the neck. Large tumors may cause cranial nerve involvement especially vagus and hypoglossal nerve.
Investigation
Investigations for paraganglioma is divided in to two broad groups.
For diagnosis
For diagnosis of glomus jugulare tumor both CT, MRI and angiogram are usually done. MRI is done for location and extent of tumor, CT scan for better assessment of the bony involvement of the skull base and angiogram for conformation of diagnosis (rule out vestibular schwannoma), ascertain patency of both jugular vein (JV). For diagnosis of carotid body tumor MRI (or CT) is used to evaluates the extent of tumor and intracranial extensions. Carotid angiogram is highly crucial to demonstrates patency of the ECA, ICA and predominant blood supply of the lesion (usually ECA with sometimes contribution from thyrocervical trunk and vertebral artery). Characteristic findingsis splaying of bifurcation.
Endocrine study
Fractionated plasma metanephrine, 24-hour urinary catecholamines and clonidine suppression test. For glomus jugulare audiometric and vestibular testing also should be performed for diagnosis of hearing loss, prognosis and medicolegal purposes.
Management
Surgery is the treatment of choice for paraganglioma. Both carotid body and glomus jugulare tumor are attached with vital neurovascular structure and there is higher chance of neurovascular injury during the surgery. Endovascular approaches are also used as solely or part of adjuvant treatment to surgery.
Medical management
Alpha and beta blockers given before surgery-blocks possibly lethal blood pressure lability and arrhythmias. Adequate blockade takes approximately 2-3 weeks of alpha blocker and at least 24 hours of beta blocker therapy; in emergency, 3 days of treatment may suffice.
Surgical management of carotid body tumor
Shamblin grouping of carotid body tumor is still widely used for surgical planning ( Table 2) Shamblin has classified the tumor into three groups/types, e.g., type 1 : tumors are small lesions, that do not splay the carotid Bifurcation; type 2 : tumors are larger, significantly splay the carotid bifurcation, but do not circumferentially encase the carotid arteries; type 3 : tumors are large, encapsulate the internal or external carotid arteries and often adhere or incorporate the adjacent cranial nerves [ 11]. Group I tumor may be accessed through a transverse incision along a cervical skin crease, giving the best cosmetic result. Larger tumors with group II or III extension require a larger exposure to control the proximal and distal carotid artery for possible shunting. Here, an oblique incision along the medial border of the sternocleidomastoid muscle is indicated. As in a standard exposure for carotid endarterectomy, the common facial vein is ligated and cut to mobilize the JV laterally. The hypoglossal and vagus nerves are identified. Frequently, the hypoglossal nerve may be displaced superiorly and posteriorly by the tumor. Consequently, care must be exerted during dissection of the superior aspect of the tumor to avoid damaging this nerve. In more challenging group III tumors, nerves may actually traverse the tumor, making dissection difficult and tedious [ 10].
Radiotherapy
The role of radiotherapy in managing carotid body tumors remains unclear [ 17].
Surgical management of glomus jugulare tumor
Due to close relation to vital neurovascular structure and relative neurovascular resistance, surgery is still the mainstay of management [ 25]. Numerous classification system has been developed for Glomus jugulare tumor, e.g., fisch classification of glomus jugulare tumors which has been classifiedn into A (tumor limited to the middle ear cleft [glomus tympanicum]); B (tumor limited to the tympanomastoid area with no involvement of the infralabyrinthine compartment); C (tumor involving the infralabyrinthine compartment of the temporal bone and extending into the petrous apex); D (tumor with intracranial extension <2 cm and >2 cm in diameter [ 8]. Tumours of type B can be tackled surgically by a tympanomastoid approach with extended facial recess dissection if indicated. It may be necessary to tie off and divide the lateral venous sinus and ligate the IJV high in the neck. Types C and D can be excised through the infra- and trans-temporal approach [ 19] or combined transmastoid retro- and infralabyrinthine transjugular transcondylar transtubercular high cervical approach [ 15]. ECA feeders are ligated early, followed rapidly by draining veins (to prevent systemic release of catecholamines). Sacrifice of the JV is tolerated if the contralateral JV is patent (often, the ipsilateral JV will already be occluded).
Role of endovascular surgery
One of the most significant advances in management has been preoperative, transvascular embolisation of these tumours.
Fractionated radiotherapy and radiosurgery
Role of radiation is still controversial and are still reserved for inoperable, surgically unsuitable, residual or in recurrent cases.
Prognosis and complication
Glomus jugulare tumors
The most common complications are cerebrospinal fluid fistula, facial nerve palsy, and varying degrees of dysphagia. Dysfunction of any of the cranial nerves VII thru XII can occur. Other possible complication include aspiration, delayed gastric emptying, ileus and excessive blood loss. Even after gross total tumor removal, recurrence rate may be as high as one third [ 12].
Carotid body tumors
Possible complication includes baroreceptor failure (33%), cranial nerve deficit (17%), vascular injury and reconstruction (28%), arterial thrombosis (7%), and death (3%) [ 11, 17].
CONCLUSION
Paraganglioma is a rare benign slow growing tumor. Concurrent carotid body and glomus jugulare tumor are extremely rare. We are reporting a case of 29-year-old male who has such type of tumor. Microsurgical resection is still the main modality of treatment. He underwent two stage surgery. Due to its rarity in nature, close proximation and adhesion with vital neurovascular structure, treatment of paraganglioma is still challenging.
Fig.┬Ā1.
Computed tomography (CT) scan showing carotid body tumor with relation to external carotid artery (ECA), internal carotid artery (ICA), and internal jugular vein. A : Contrast CT. B : Non-contrast CT. CT scan of cranial base showing left glomus jugulare tumor with intra (C) and extra (D) cranial extension. 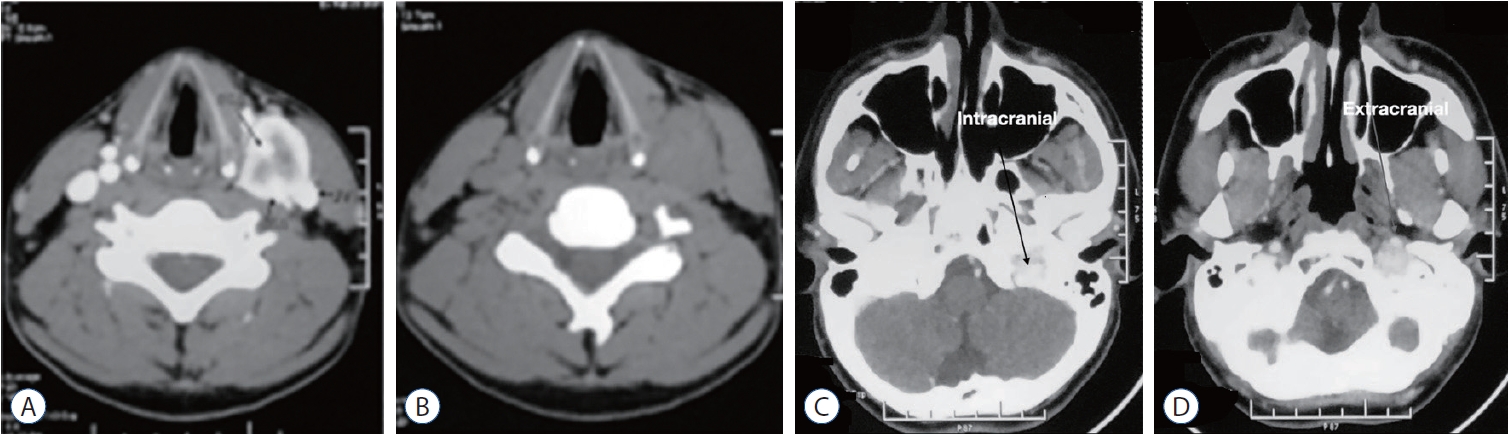
Fig.┬Ā2.
Magnetic resonance imaging (MRI) of brain showing left glomus jugular in different phase of acquisition (marked with arrow). Vascular flow void present withing the lesion. 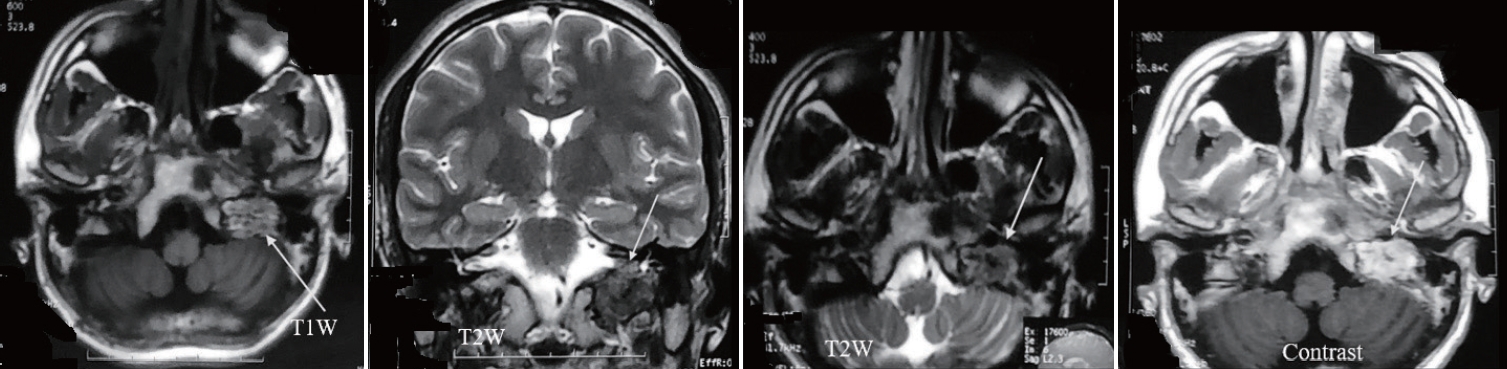
Fig.┬Ā3.
Computed tomography (CT) angiogram of the neck vessels showing carotid body tumor with relation to left common carotid artery, external carotid artery (ECA), and internal carotid artery (ICA). Left ECA is engulfed by the tumor (A and B) magnetic resonance venogram of the cranial vessels. Showing occlusion of left internal jugular vein (arrow marked; C and D). Lt : left. 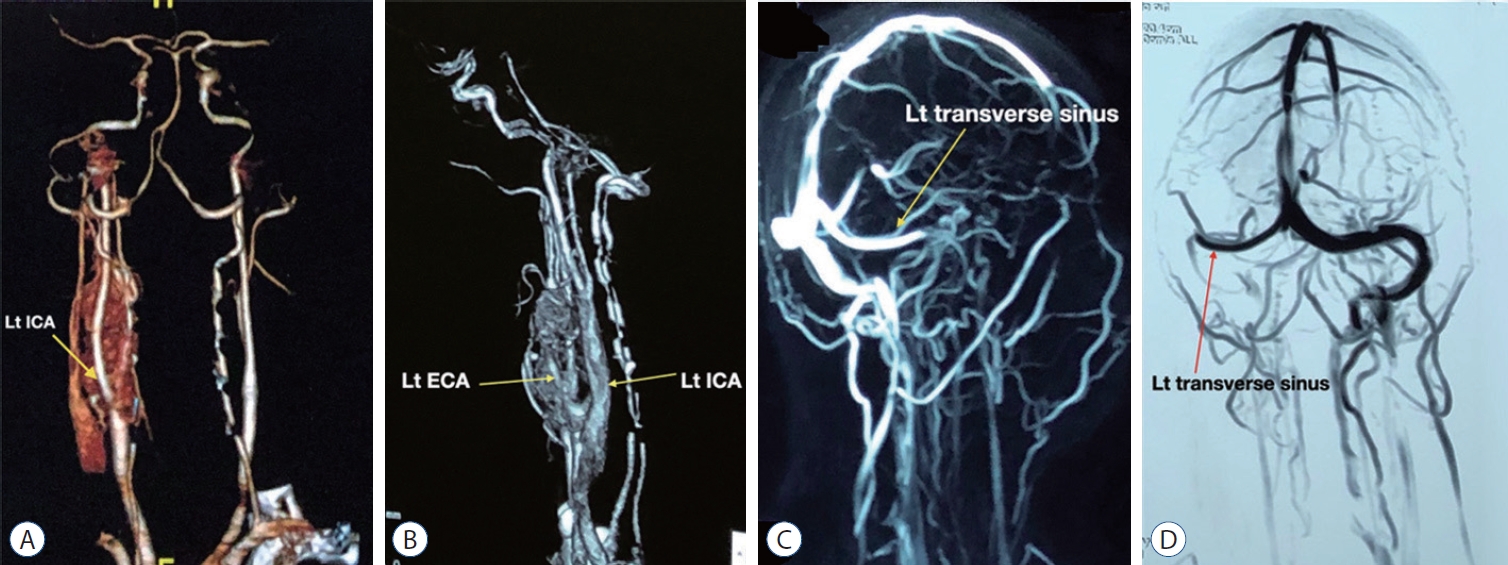
Fig.┬Ā4.
Incision marking for removal of the carotid body tumor (A), dissection at different stage around internal carotid artery (B-D). 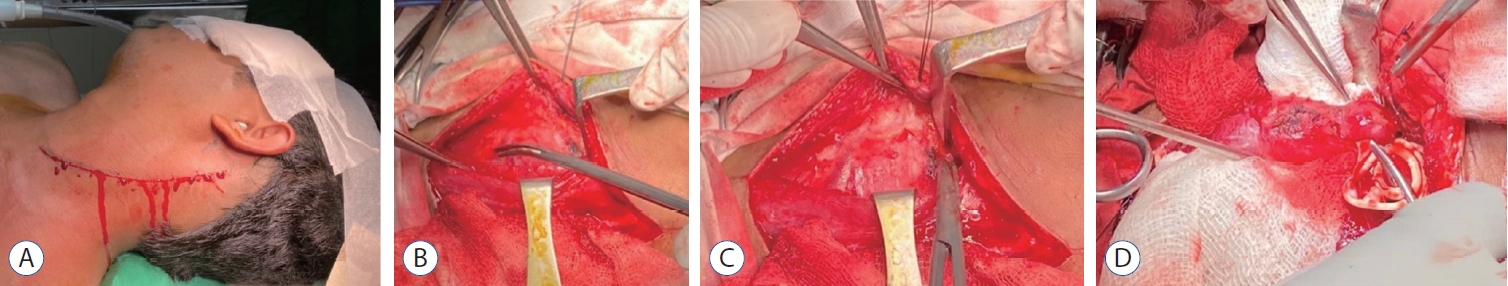
Fig.┬Ā5.
Incision marking for glomus jugulare tumor with scar of previous surgery. 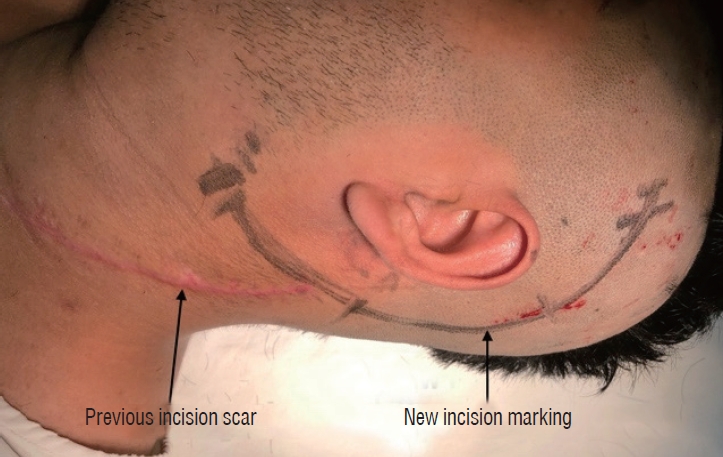
Table┬Ā1.
Paraganglioma designation based on site of origin [ 16]
|
Site |
Designation |
|
Carotid bifurcation (most common) |
Carotid body tumors |
|
Auricular branch of vagus (middle ear) |
Glomus tympanicum |
|
Superior vagal ganglion (jugular foramen) |
Glomus jugulare |
|
Inferior vagal ganglion (nasopharynx at skull base) (least common) |
Glomus intervagale |
|
Adrenal medulla and sympathetic chain |
Pheochromocytoma |
Table┬Ā2.
Shamblin classification of carotid body tumors [ 11]
|
Type 1 |
Tumors are small lesions, that do not splay the carotid bifurcation |
|
Type 2 |
Tumors are larger, significantly splay the carotid bifurcation, but do not circumferentially encase the carotid arteries |
|
Type 3 |
Tumors are large, encapsulate the internal or external carotid arteries and often adhere or incorporate the adjacent cranial nerves |
References
1. Alexander MJ, Moftakhar P : Nonatherosclerotic Carotid Lesions Emobolization in Winn HR (ed) : Youmans Neurological Surgery EBook. Philadelphia : Elsevier Health Sciences, 2011, pp3655-3664
2. Ammirati M, Zarzour H : Overview of Skull Base Tumors in Winn HR (ed) : Youmans Neurological Surgery E-Book. Philadelphia : Elsevier Health Sciences, 2011, pp1569-1586
3. Askenasy HM, Eppenstein SS, Herzberger EE : Tumor of the glomus jugulare co-existing possibly with carotid body tumor. Acta Neurochir (Wien) 3 : 170-179, 1953    4. Ball JR, Hurlbert RJ : Endovascular Techniques for Tumor Emobolization in Winn HR (ed) : Youmans Neurological Surgery E-Book. Philadelphia : Elsevier Health Sciences, 2011, pp1226,
5. Bogdasarian RS, Lotz PR : Multiple simultaneous paragangliomas of the head and neck in association with multiple retroperitoneal pheochromocytomas. Otolaryngol Head Neck Surg (1979) 87 : 648-652, 1979    6. Casterline PF, Jaques DA : Simultaneous recurrent multiple chemodectomas. Arch Otolaryngol 104 : 157-160, 1978   7. Farrior JB 3rd, Hyams VJ, Benke RH, Farrior JB : Carcinoid apudoma arising in a glomus jugulare tumor: review of endocrine activity in glomus jugulare tumors. Laryngoscope 90 : 110-119, 1980   8. Gerosa M, Paolo R : Glomus Tumors in Winn HR (ed) : Youmans Neurological Surgery E-Book. Philadelphia : Elsevier Health Sciences, 2011, pp1594-1609
9. Green JD Jr, Brackmann DE, Nguyen CD, Arriaga MA, Telischi FF, De la Cruz A : Surgical management of previously untreated glomus jugulare tumors. Laryngoscope 104( 8 Pt 1):917-921, 1994   10. Greenberg MS : Handbook of neurosurgery. Stuttgart : Thieme, 2019
12. Islam MR, Ansari A, Rahman A, Saklayen SMG, Muhammad N, Shah SK, et al : The perplexing postsurgical complication of carotid-jugular fistula: a bitter experience. Surg Neurol Int 13 : 2, 2022    13. Jackson CG, Harris PF, Glasscock ME 3rd, Fritsch M, Dimitrov E, Johnson GD, et al : Diagnosis and management of paragangliomas of the skull base. Am J Surg 159 : 389-393, 1990   14. Klingbeil JR : Multiplicity and familial incidence of carotid body and glomus jugulare tumors. Plas Reconst Surg 38 : 489, 1966  15. Liu JK, Sameshima T, Gottfried ON, Couldwell WT, Fukushima T : The combined transmastoid retro- and infralabyrinthine transjugular transcondylar transtubercular high cervical approach for resection of glomus jugulare tumors. Neurosurgery 59( 1 Suppl 1):ONS-115-25discussion ONS115-25, 2006  16. Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, Granados-Garcia M, Herrera-Gomez A : Carotid body tumors: review of a 20-year experience. Oral Oncol 41 : 56-61, 2005   17. Mafee MF, Raofi B, Kumar A, Muscato C : Glomus faciale, glomus jugulare, glomus tympanicum, glomus vagale, carotid body tumors, and simulating lesions. Role of MR imaging. Radiol Clin North Am 38 : 1059-1076, 2000  19. Moffat DA, Hardy DG : Surgical management of large glomus jugulare tumours: infra- and trans-temporal approach. J Laryngol Otol 103 : 1167-1180, 1989   20. Pareschi R, Righini S, Destito D, Raucci AF, Colombo S : Surgery of glomus jugulare tumors. Skull Base 13 : 149-157, 2003    21. Parkinson D : Intracranial pheochromocytomas (active glomus jugulare). Case report. J Neurosurg 31 : 94-100, 1969  22. Sessions RT, McSwain B, Carlson RI, Scott HW Jr : Surgical experiences with tumors of the carotid body, glomus jugulare and retroperitoneal nonchromaffin paraganglia. Ann Surg 150 : 808-823, 1959    23. Thompson JW, Cohen SR : Management of bilateral carotid body tumors and a glomus jugulare tumor in a child. Int J Pediatr Otorhinolaryngol 17 : 75-87, 1989   24. Thompson JW, Cohen SR : Management of bilateral carotid body tumors and a glomus jugulare tumor in a child. Int J Pediatr Otorhinolaryngol 17 : 75-87, 1989   25. Watkins LD, Mendoza N, Cheesman AD, Symon L : Glomus jugulare tumours: a review of 61 cases. Acta Neurochir (Wien) 130 : 66-70, 1994    26. Young WF Jr : Paragangliomas: clinical overview. Ann N Y Acad Sci 1073 : 21-29, 2006   27. Zacks SI : Chemodectomas occurring concurrently in the neck (carotid body), temporal bone (glomus jugulare) and retroperitoneum; report of a case with histochemical observations. Am J Pathol 34 : 293, 1958  
|
|





















