Lee and Lim: Monocyte Count and Systemic Immune-Inflammation Index Score as Predictors of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage
Abstract
Objective
Delayed cerebral ischemia (DCI) is a major cause of disability in patients who survive aneurysmal subarachnoid hemorrhage (aSAH). Systemic inflammatory markers, such as peripheral leukocyte count and systemic immune-inflammatory index (SII) score, have been considered predictors of DCI in previous studies. This study aims to investigate which systemic biomarkers are significant predictors of DCI.
Methods
We conducted a retrospective, observational, single-center study of 170 patients with SAH admitted between May 2018 and March 2022. We analyzed the patients’ clinical and laboratory parameters within 1 hour and 3-4 and 5-7 days after admission. The DCI and non-DCI groups were compared. Variables showing statistical significance in the univariate logistic analysis (p<0.05) were entered into a multivariate regression model.
Results
Hunt-Hess grade “4-5” at admission, modified Fisher scale grade “3-4” at admission, hydrocephalus, intraventricular hemorrhage, and infection showed statistical significance (p<0.05) on a univariate logistic regression. Lymphocyte and monocyte count at admission, SII scores and C-reactive protein levels on days 3-4, and leukocyte and neutrophil counts on days 5-7 exhibited statistical significance on the univariate logistic regression. Multivariate logistic regression analysis revealed that monocyte count at admission (odds ratio [OR], 1.64; 95% confidence interval [CI], 1.04-2.65; p=0.036) and SII score at days 3-4 (OR, 1.55; 95% CI, 1.02-2.47; p=0.049) were independent predictors of DCI.
Conclusion
Monocyte count at admission and SII score 3-4 days after rupture are independent predictors of clinical deterioration caused by DCI after aSAH. Peripheral monocytosis may be the primer for the innate immune reaction, and the SII score at days 3-4 can promptly represent the propagated systemic immune reaction toward DCI.
Key Words: Monocytes · Systemic immune-inflammation index score · Delayed cerebral ischemia · Aneurysmal subarachnoid hemorrhage.
INTRODUCTION
Despite advances in specialized management strategies and resources for neurointensive care units, aneurysmal subarachnoid hemorrhage (aSAH) remains one of the most fatal cerebrovascular diseases [ 5, 10]. Following aSAH, cerebral vasospasm is a possible cause of disability in those who survive the initial hemorrhage [ 2, 3]. Cerebral vasospasm is primarily responsible for the secondary ischemia (or delayed ischemic neurological deficit), which occurs in approximately 30% of patients. Clazosentan, an endothelin-1 receptor antagonist, is a novel pharmacological agent that effectively decreases and reverses cerebral vasospasm in the large major arteries. However, the clazosentan in Aneurysmal Subarachnoid Hemorrhage trial, which is a randomized trial of clazosentan in patients with aSAH, failed to show improved outcomes, despite clazosentan’s ability to reduce radiographic vasospasm [ 13, 14]. Therefore, the clinical definition of delayed cerebral ischemia (DCI) proposed by a multidisciplinary research group in 2010 is considered to be more useful for predicting outcomes in patients with aSAH than traditional “radiographic vasospasm.” DCI is currently defined by two main outcome measures : 1) cerebral infarction identified on computed tomography (CT) or magnetic resonance imaging (MRI) or confirmed at autopsy and 2) clinical deterioration with the occurrence of focal neurological impairment or a decrease of at least 2 points on the Glasgow coma scale, after exclusion of other causes [ 23]. DCI is caused by multifactorial pathophysiologies, such as large artery vasospasm, microvascular dysfunction, microthrombosis, neuroinflammation, and/or excitotoxicity. These are known to be triggered by initial injury to the brain and propagated through mechanisms related to oxyhemoglobin, released by erythrolysis, 2 days after SAH. Neuroinflammation and microthrombosis are considered key factors for the production of vasoactive agents and circulatory disturbances [ 7, 15, 16, 21]. Systemic inflammatory markers, such as peripheral leukocyte count, have been introduced as predictive factors for DCI [ 1, 9, 20]. Mean platelet volume (MPV) and platelet counts (PCs) are considered to reflect microthrombosis [ 18, 24]. However, some of the previous studies are still based on the traditional concept of “vasospasm.” In addition, there is a lack of consistency among studies in which the laboratory test results are reliable. Recently, the systemic immune-inflammation index (SII) score at admission has been introduced to predict the outcomes of various diseases [ 19, 25]. Some studies have evaluated the role of the Systemic immune-inflammatory index (SII) from blood count at admission on predicting radiographic vasospasm or DCI [ 4, 8]. Thus, we sampled complete blood counts and C-reactive protein (CRP) levels on admission before treatment, 3-4 days after admission, and 5-7 days after admission to verify which factors are reliable for predicting DCI for patients with aSAH.
MATERIALS AND METHODS
Study design and patient population
This was a retrospective, observational, single-center study of patients with SAH admitted between May 2018 and March 2022 to a tertiary care hospital located in Republic of Korea. This study was approved by the Ajou University Medical Center Institutional Review Board (AJOUIRB-DB-2022-558).
In total, 244 patients with SAH were identified based on the International Classification of Diseases, Tenth Revision (I60.XX) codes. Patients aged <18 years, who were unable to be followed up for 7 days, who were admitted to the hospital after 24 hours of bleed ictus, and whose SAH was associated with arteriovenous malformation, intracranial arterial dissection, mycotic or traumatic aneurysm, or infectious or autoimmune disease at admission were excluded from this study. Aneurysmal SAH was confirmed using conventional angiography, and all aneurysms were treated with either surgical clipping or endovascular treatment.
Demographics and clinical parameters
The baseline characteristics of the subjects were identified from electronic medical records. The clinical variables included age, sex, cardiovascular risk factors (hypertension, diabetes mellitus), aneurysm location (anterior or posterior circulation), treatment modality (clip or coil), and presence of seizures. Neurological status at presentation was assessed using the Hunt-Hess grade (HHG). Radiographic characteristics at admission were assessed using the modified Fisher scale (mFS) and presence of hydrocephalus, intracerebral hemorrhage (ICH), or intraventricular hemorrhage (IVH). HHG and mFS were dichotomized for analytical purposes as follows : HHG (“1-3” and “4-5”) and mFS (“1-2” and “3-4”). Infection was defined as newly developed infectious diseases after admission, such as urinary tract infection, pneumonia, or blood stream bacteremia.
Laboratory parameters
We analyzed the laboratory parameters within 1 hour and 3-4 days and 5-7 days after admission. Blood specimens were analyzed using a Beckman Coulter LH 780 (Beckman Coulter, Miami, FL, USA) within 30 minutes after venipuncture. Complete blood counts, white blood cell differential counts, MPVs, and CRP levels were evaluated. The ratio of MPV to PC ([MPV / PC] × 100) was used as a surrogate marker for the level of platelet activation. The SII score was calculated as follows : PC × neutrophil count / lymphocyte count.
Definition and assessment of DCI
The current clinical definition of DCI was applied in this study : 1) cerebral infarction identified on CT or MRI or confirmed at autopsy and 2) clinical deterioration with occurrence of focal neurological impairment or a decrease of at least 2 points on the Glasgow coma scale, after exclusion of other causes. Focal neurological impairment includes hemiparesis, hemiparesthesia, aphasia, apraxia, neglect, and hemianopia. Deficits presented immediately after the aneurysm treatment was excluded. The presence of DCI was identified by reviewing the patients’ electronic medical records from the consensus of three neurosurgeons.
Statistical analyses
Statistical analyses were performed using the R software version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). The baseline clinical characteristics between the DCI and non-DCI groups were compared using the chisquared or Student’s t-test, as appropriate. Variables showing significance in the univariate logistic analysis (p<0.05) for predicting DCI were entered into a multivariate regression model. Collinearity among variables was also considered in the selection of variables. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were established to determine the ability of the variables to predict DCI. The descriptive performance for each variable was compared using the DeLong test.
RESULTS
A total of 170 (105 female [62.0%]; 65 male [38.0%]) patients with aneurysmal SAH met the inclusion criteria ( Fig. 1). The median age at the time of admission was 56 years (range, 45-63). History of hypertension or diabetes mellitus was observed in 61 (36.0%) and 16 patients (9.4%), respectively. Hydrocephalus, IVH, and ICH on the initial radiographic imaging were observed in 73 (43.0%), 39 (23.0%), and 32 patients (19.0%), respectively. Most ruptured aneurysms were located in the anterior circulation (94.0%) and were treated with either clipping (24.0%) or coiling (76.0%). Infectious diseases, such as aspiration pneumonia, which developed during the follow-up period and were not present at admission, were documented in 32 patients (19.0%). In total, 42 patients (25.0%) had HHGs ranging from “4 to 5”, whereas 141 patients (83.0%) had mFS grades ranging from “3 to 4.” DCI was observed in 38 patients (22.0%) ( Table 1). All patients were assigned into two groups based on the occurrence of DCI. DCI was significantly correlated with HHG “4-5” at admission (19% vs. 45%, p=0.001) and with mFS grade “3-4” at admission (80% vs. 95%, p=0.028). Patients with DCI tended to have higher incidence rates of hydrocephalus (37% vs. 63%, p=0.004) and IVH (16% vs. 47%, p<0.001) than those without DCI. In addition, some laboratory variables showed significant differences between the patients with and without DCI ( Table 1). Univariate logistic regression analysis was performed to evaluate the value of each parameter as a predictor of DCI. Among the clinical parameters, HHG “4-5” at admission, mFS grade “3-4” at admission, hydrocephalus, IVH, and infection showed statistical significance ( p<0.05). Among the laboratory parameters, lymphocyte and monocyte counts at admission were statistically significant ( p=0.026 and p<0.001, respectively). At days 3-4, leukocyte count, neutrophil count, neutrophil-to-lymphocyte ratio, SII score, and CRP level were statistically significant ( p=0.005, p=0.004, p=0.005, p=0.002, and p=0.048, respectively). At days 5-7, leukocyte and neutrophil counts were statistically significant ( p=0.027 and p=0.017, respectively) ( Table 2). Parameters with p-value <0.05, as determined by univariate analysis, were selected for the multivariate analysis, and collinearity among the parameters was considered. Multivariate logistic regression analysis showed that monocyte count at admission (odds ratio [OR], 1.64; 95% confidence interval [CI], 1.04-2.65; p=0.036) and SII score at days 3-4 (OR, 1.55; 95% CI, 1.02-2.47; p=0.049) were independent risk factors for DCI ( Table 3). ROC curves were demonstrated with monocyte count at admission, systemic inflammatory index score at day 3-4, and a combination of the two parameters ( Fig. 2). Each showed AUCs of 0.63 (95% CI, 0.53-0.74), 0.70 (95% CI, 0.60-0.80), and 0.75 (95% CI, 0.66-0.83) respectively, without statistically significant different on DeLong’s test ( Table 4).
DISCUSSION
DCI is considered an important determinant of poor neurological outcomes in patients who survive aSAH. The pathophysiology of DCI is multifactorial, including large artery vasospasm, microvascular dysfunction, microthrombosis, and neuroinflammation, among which neuroinflammation and microthrombosis are the leading factors in the propagation of devastating microenvironment and neuronal injury.
Laboratory data from blood samples can reflect the underlying pathogenesis and be used as surrogates for samples from the central nervous system. Several systemic biomarkers, such as peripheral leukocytosis and differential counts, have been studied as predictive markers of DCI [ 7, 15, 16, 20]. Particularly, peripheral monocytosis at admission has been an important factor for cerebral infarction and poor functional outcomes in patients with SAH [ 22]. Moreover, the systemic inflammatory index, which comprehensively reflects the activity of neutrophils and platelets, is a powerful predictor of DCI [ 18, 24]. However, such parameters have been studied individually and generally measured at admission; therefore, the association between parameters and time-dependent effects on DCI has not been sufficiently evaluated. Therefore, we sampled blood at admission, 3-4 days after the ictus (shortly before the DCI period), and 5-7 days after the ictus (initial DCI period) and compared the predictive value of each biomarker. In the present study, clinical predictors, such as initial HHG, mFS grade, hydrocephalus, and IVH, had statistically significant predictive value for DCI, consistent with the result of a previous study [ 11]. Infection, defined as newly developed infectious diseases after admission, such as urinary tract infection, pneumonia, or blood stream bacteremia, also showed predictive value in univariate analysis ( Table 2). However, when multivariate analysis was performed, clinical predictors were found to have no statistical significance. Two laboratory parameters that were shown to be statistically significant were monocyte count at admission and SII score on day 3-4 after the ictus ( Table 3). The result of the current study supports the findings of previous studies and presents a perspective that temporal information should also be considered. In the present study, inf lammatory markers positively correlated with DCI. In particular, the monocyte count at admission showed statistical significance even after adjustment for other parameters, which is consistent with the result of a previous study [ 22]. However, the SII score showed predictive value only on days 3-4, not at admission or days 5-7, which was different from the findings of previous studies [ 4, 8]. DCI has distinct property in that it develops in delayed manner. Pathophysiological factors, such as oxyhemoglobin, reactive oxygen species, and pro-inflammatory cytokines, need sufficient time to be propagated [ 21]. Considering the immunological process from the activation of innate immunity to adaptive immunity, our data seem plausible. Peripheral monocytes with increased expression of toll-like receptor 4 (TLR4) correlate with poor clinical presentation and development of DCI [ 8]. TLR4 is an immune activator that increases the expression of interleukin (IL)-1, IL-6, tumor necrosis factor-α, and monocyte chemoattractant protein-1 [ 12]. Peripheral monocytes expressing CC chemokine receptor 2 are thought to be recruited to the central nervous system by the interaction between endothelial cells through CC chemokine ligand 2 [ 6]. Subsequently, neuroinflammation may result in blood-brain barrier disruption, causing pro-inflammatory factors to be released into the systemic circulation, which leads to the activation of the systemic inflammatory response [ 17]. In this perspective, the pathogenesis of DCI can be initially represented by an increased monocyte count, and the propagation of the immune response can be represented by the SII. We hypothesize that inflammatory biomarkers, such as monocyte count and SII score, can be helpful in neurocritical care to estimate a patient’s risk of DCI and to determine management strategies, such as induced hypertension or angioplasty. Temporal information should be considered when interpreting and applying inflammatory markers to predict DCI. Although complex biochemical mechanisms have not been directly identified, our study suggests that attention should be paid to the progress of the immunological process to reveal the mechanisms of DCI.
However, our study has some limitations. First, this was a single-center, observational study. Therefore, there is a possibility of bias, and the findings may not be generalizable to other clinical settings. Second, we could not identify the exact mechanism by which initial peripheral monocytosis progressed toward the propagation of systemic inflammation. Third, additional management-associated factors, such as total cumulative dose of nimodipine, induced hypertension, level of sedation, or therapeutic hypothermia, were not included in the analysis. Finally, we did not evaluate the functional outcomes in patients with monocytosis or high surgical site infection. A multicenter prospective study can verify the causality between immunologic biomarkers and DCI, and further experimental studies are required to identify the detailed mechanisms of DCI.
CONCLUSION
Monocyte count at admission and SII score 3-4 days after rupture are independent predictors of DCI after aSAH. Peripheral monocytosis may be the primer for the innate immune reaction, and SII score on days 3-4 can promptly represent the propagated systemic immune reaction toward DCI.
Fig. 1.
Study design with inclusion and exclusion criteria for aSAH patients. aSAH : aneurysmal subarachnoid hemorrhage, AVM : arteriovenous malformation, DCI : delayed cerebral ischemia. 
Fig. 2.
Receiver operating characteristic curves to identify patients with delayed cerebral ischemia. SII : Systemic immune-inflammatory index. 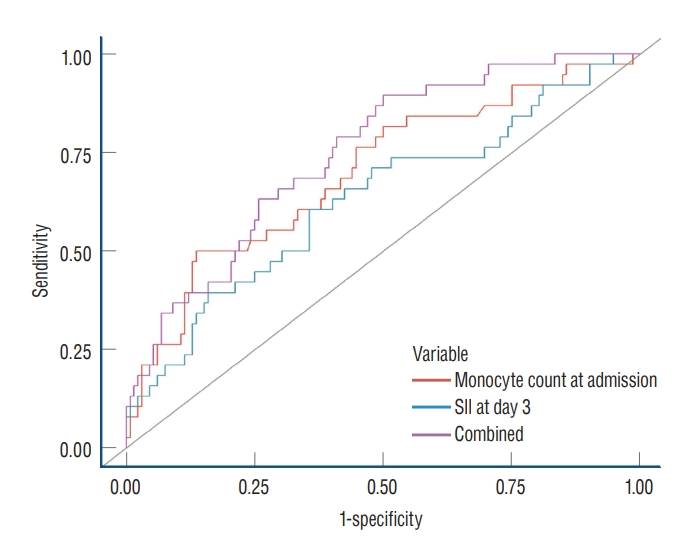
Table 1.
Baseline characteristics of aSAH patients with and without delayed cerebral ischemia
|
Total (n=170) |
DCI
|
p-value |
|
Yes (n=38) |
No (n=132) |
|
Clinical variable |
|
|
|
|
|
Age (years) |
56 (45-63) |
52 (44-64) |
56 (46-62) |
0.5 |
|
Sex, female |
105 (62.0) |
24 (63.0) |
81 (61.0) |
0.8 |
|
Hunt-Hess grade |
|
|
|
0.001 |
|
<4 |
128 (75.0) |
21 (55.0) |
107 (81.0) |
|
|
≥4 |
42 (25.0) |
17 (45.0) |
25 (19.0) |
|
|
Modified Fisher scale |
|
|
|
0.028 |
|
<3 |
29 (17.0) |
2 (5.3.0) |
27 (20.0) |
|
|
≥3 |
141 (83.0) |
36 (95.0) |
105 (80.0) |
|
|
Hydrocephalus |
73 (43.0) |
24 (63.0) |
49 (37.0) |
0.004 |
|
IVH |
39 (23.0) |
18 (47.0) |
21 (16.0) |
<0.001 |
|
ICH |
32 (19.0) |
9 (24.0) |
23 (17.0) |
0.4 |
|
Aneurysm location |
|
|
|
0.7 |
|
Anterior |
160 (94.0) |
35 (92.0) |
125 (95.0) |
|
|
Posterior |
10 (59.0) |
3 (79.0) |
7 (53.0) |
|
|
Treatment modality |
|
|
|
0.2 |
|
Clip |
41 (24.0) |
6 (16.0) |
35 (27.0) |
|
|
Coil |
129 (76.0) |
32 (84.0) |
97 (73.0) |
|
|
Infection |
32 (19.0) |
12 (32.0) |
20 (15.0) |
0.022 |
|
HTN |
61 (36.0) |
14 (37.0) |
47 (36.0) |
0.9 |
|
DM |
16 (94.0) |
3 (79.0) |
13 (98.0) |
0.9 |
|
Seizure |
17 (10.0) |
6 (16.0) |
11 (8.3.0) |
0.2 |
|
Laboratory variable |
|
|
|
|
|
At admission |
|
|
|
|
|
Leukocyte count (103/µL) |
10.8 (8.6-14.5) |
10.6 (8.6-14.6) |
10.8 (8.6-14.4) |
0.8 |
|
Neutrophil count (103/µL) |
8.56 (5.8-11.65) |
7.47 (5.28-12.27) |
8.62 (5.95-1.165) |
0.5 |
|
Lymphocyte count (103/µL) |
1.51 (0.99-2.24) |
1.99 (1.31- 2.93) |
1.40 (0.96-2.03) |
0.009 |
|
Monocyte count (103/µL) |
0.55 (0.40-0.72) |
0.70 (0.54-0.93) |
0.53 (0.38-0.65) |
<0.001 |
|
NLR |
5.7 (2.8-9.5) |
5.3 (1.8-8.3) |
5.8 (3.1-9.9) |
0.091 |
|
Platelet count (103/µL) |
233 (199-274) |
256 (183-279) |
232 (201-268) |
0.5 |
|
Hemoglobin (g/dL) |
13.5 (12.62-14.70) |
13.90 (13.03-15.10) |
13.50 (12.47-14.53) |
0.1 |
|
MPV (fL) |
8.00 (7.53-8.60) |
8.05 (7.70-8.90) |
8.00 (7.50-8.53) |
0.4 |
|
MPV/PC |
3.4 (2.9-4.2) |
3.2 (2.8-4.4) |
3.4 (2.9-4.1) |
0.7 |
|
SII |
1272 (643-2296) |
1125 (415-2218) |
1328 (685-2356) |
0.2 |
|
CRP (mg/dL) |
0.14 (0.06- 0.38) |
0.09 (0.05-0.28) |
0.17 (0.06-0.41) |
0.3 |
|
At days 3-4 |
|
|
|
|
|
Leukocyte count |
11.2 (9.2-14.6) |
13.8 (10.8-16.1) |
10.9 (8.9-13.4) |
<0.001 |
|
Neutrophil count |
8.98 (6.80-11.65) |
11.06 (8.34-13.50) |
8.31 (6.51-11.20 |
<0.001 |
|
Lymphocyte count |
1.38 (1.04-1.86) |
1.27 (0.9-1.83) |
1.41 (1.08-1.86) |
0.2 |
|
Monocyte count |
0.72 (0.52-0.94) |
0.80 (0.58-1.05) |
0.70 (0.50-0.89) |
0.081 |
|
NLR |
6.3 (4.3-10.8) |
8.4 (5.8-13.1) |
5.8 (4.1-10.2) |
0.002 |
|
Platelet count |
214 (180 - 247) |
215 (183-244) |
214 (180-248) |
0.9 |
|
Hemoglobin |
12.10 (10.62-13.30) |
11.70 (10.80-13.25) |
12.20 (10.50-13.43) |
0.9 |
|
MPV |
8.20 (7.70-8.70) |
8.40 (7.80-8.90) |
8.15 (7.68-8.70) |
0.2 |
|
MPV/PC |
3.9 (3.2-4.8) |
4.0 (3.3-4.9) |
3.8 (3.2-4.8) |
0.4 |
|
SII |
1306 (874-2494) |
1666 (970-2991) |
1,163 (841-2,178) |
0.013 |
|
CRP |
1.4 (0.3-4.4) |
3.8 (1.0-6.7) |
1.2 (0.3-3.2) |
0.005 |
|
At days 5-7 |
|
|
|
|
|
Leukocyte count |
10.2 (7.9-12.8) |
11.8 (8.0-13.9) |
9.9 (7.9-12.3) |
0.085 |
|
Neutrophil count |
6.9 (5.29-9.76) |
8.95 (5.68-10.95) |
6.86 (5.27-9.33) |
0.044 |
|
Lymphocyte count |
1.58 (1.13-2.07) |
1.52 (1.10-2.15) |
1.59 (1.17-2.07) |
0.6 |
|
Monocyte count |
0.79 (0.56-1.02) |
0.77 (0.56-1.02) |
0.80 (0.56-1.02) |
0.7 |
|
NLR |
4.6 (2.8-7.8) |
6.0 (3.7-9.4) |
4.2 (2.6-6.6) |
0.032 |
|
Platelet count |
218 (178-262) |
219 (176-266) |
218 (179-259) |
0.9 |
|
Hemoglobin |
11.3 (10.10-12.40) |
11.55 (9.95-12.17) |
11.30 (10.10-12.45) |
0.9 |
|
MPV |
8.00 (7.50-8.57) |
8.05 (7.50-8.60) |
8.00 (7.50-8.50) |
0.7 |
|
MPV/PC |
3.7 (3.0-4.6) |
3.7 (2.9-4.6) |
3.7 (3.0-4.7) |
0.9 |
|
SII |
991 (584-1675) |
1349 (619-2044) |
874 (576-1602) |
0.072 |
|
CRP |
1.3 (0.6-2.8) |
1.9 (1.0-4.3) |
1.1 (0.5-2.5) |
0.011 |
Table 2.
Univariate logistic regression analysis to predict delayed cerebral ischemia
|
Univariate analysis
|
|
Odds ratio |
95% confidence interval |
p-value |
|
Clinical parameter |
|
|
|
|
Age |
0.99 |
0.96-1.02 |
0.4 |
|
Sex |
|
|
|
|
Female |
- |
|
- |
|
Male |
0.93 |
0.43-1.94 |
0.8 |
|
Hunt- Hess grade |
|
|
|
|
<4 |
- |
|
- |
|
≥4 |
3.46 |
1.59-7.55 |
0.002 |
|
Modified Fisher scale |
|
|
|
|
<3 |
- |
|
- |
|
≥3 |
4.63 |
1.30-29.6 |
0.04 |
|
Hydrocephalus |
2.90 |
1.39-6.26 |
0.005 |
|
IVH |
4.76 |
2.17-10.6 |
<0.001 |
|
ICH |
1.47 |
0.59-3.44 |
0.4 |
|
Aneurysm location |
|
|
|
|
Anterior |
- |
|
- |
|
Posterior |
1.53 |
0.32-5.82 |
0.6 |
|
Treatment modality |
|
|
|
|
Clip |
- |
|
- |
|
Coil |
1.92 |
0.79-5.45 |
0.2 |
|
Infection |
2.58 |
1.10-5.92 |
0.026 |
|
HTN |
1.05 |
0.49-2.21 |
0.9 |
|
DM |
0.78 |
0.17-2.60 |
0.7 |
|
Seizure |
2.06 |
0.67-5.86 |
0.2 |
|
Laboratory parameter |
|
|
|
|
At admission |
|
|
|
|
Leukocyte count |
1.19 |
0.83-1.68 |
0.3 |
|
Neutrophil count |
1.01 |
0.69-1.43 |
1.0 |
|
Lymphocyte count |
1.46 |
1.04-2.05 |
0.026 |
|
Monocyte count |
2.08 |
1.45-3.09 |
<0.001 |
|
NLR |
0.86 |
0.54-1.24 |
0.5 |
|
Platelet count |
1.17 |
0.82-1.68 |
0.4 |
|
Hemoglobin |
1.35 |
0.93-2.02 |
0.1 |
|
MPV |
1.15 |
0.80-1.65 |
0.4 |
|
MPV/PC |
0.92 |
0.60-1.32 |
0.7 |
|
SII |
0.97 |
0.63-1.37 |
0.9 |
|
CRP |
1.00 |
0.66-1.40 |
1.0 |
|
At days 3-4 |
|
|
|
|
Leukocyte count |
1.84 |
1.25-2.89 |
0.005 |
|
Neutrophil count |
1.86 |
1.26-2.91 |
0.004 |
|
Lymphocyte count |
0.80 |
0.53-1.15 |
0.2 |
|
Monocyte count |
1.00 |
0.60-1.38 |
1.0 |
|
NLR |
1.68 |
1.20-2.51 |
0.005 |
|
Platelet count |
1.01 |
0.70-1.45 |
0.9 |
|
Hemoglobin |
1.11 |
0.77-1.60 |
0.6 |
|
MPV |
1.31 |
0.91-1.87 |
0.1 |
|
MPV/PC |
1.11 |
0.77-1.58 |
0.6 |
|
SII |
1.78 |
1.25-2.69 |
0.002 |
|
CRP |
1.38 |
1.00-1.95 |
0.048 |
|
At days 5-7 |
|
|
|
|
Leukocyte count |
1.48 |
1.05-2.13 |
0.027 |
|
Neutrophil count |
1.53 |
1.08-2.18 |
0.017 |
|
Lymphocyte count |
0.95 |
0.65-1.36 |
0.8 |
|
Monocyte count |
0.98 |
0.68-1.40 |
0.9 |
|
NLR |
1.25 |
0.89-1.76 |
0.2 |
|
Platelet count |
1.11 |
0.77-1.59 |
0.6 |
|
Hemoglobin |
0.98 |
0.68-1.41 |
0.9 |
|
MPV |
1.13 |
0.79-1.62 |
0.5 |
|
MPV/PC |
0.98 |
0.61-1.37 |
0.9 |
|
SII |
1.36 |
0.96-1.90 |
0.077 |
|
CRP |
1.30 |
0.93-1.79 |
0.1 |
Table 3.
Multivariate logistic regression analysis to predict delayed cerebral ischemia
|
Parameter |
Multivariate analysis
|
|
Odds ratio |
95% confidence interval |
p-value |
|
Hunt-Hess grade |
|
|
0.5 |
|
<4 |
- |
|
- |
|
≥4 |
1.16 |
0.40-3.27 |
0.8 |
|
Modified Fisher scale |
|
|
|
|
<3 |
- |
|
- |
|
≥3 |
1.96 |
0.47-13.4 |
0.4 |
|
Hydrocephalus |
1.15 |
0.41-3.14 |
0.8 |
|
IVH |
2.14 |
0.74-6.09 |
0.2 |
|
Infection |
1.18 |
0.36-3.73 |
0.8 |
|
At admission |
|
|
|
|
Lymphocyte count |
1.15 |
0.78-1.70 |
0.5 |
|
Monocyte count |
1.64 |
1.04-2.65 |
0.0 |
|
At days 3-4 |
|
|
|
|
SII |
1.55 |
1.02-2.47 |
0.049 |
|
CRP |
0.95 |
0.60-1.42 |
0.8 |
|
At days 5-7 |
|
|
|
|
Neutrophil count |
0.85 |
0.52-1.36 |
0.5 |
Table 4.
Predictive values of monocyte count and SII for delayed cerebral ischemia
|
Parameter |
AUC |
95% confidence interval |
p-value |
|
Combined |
0.75 |
0.66-0.83 |
Reference |
|
Monocyte count at admission |
0.70 |
0.60-0.80 |
0.189 |
|
SII at days 3-4 |
0.63 |
0.53-0.74 |
0.015 |
References
1. Bacigaluppi S, Ivaldi F, Bragazzi NL, Benvenuto F, Gallo F, D’Andrea A, et al : An early increase of blood leukocyte subsets in aneurysmal subarachnoid hemorrhage is predictive of vasospasm. Front Neurol 11 : 587039, 2020    2. Balança B, Bouchier B, Ritzenthaler T : The management of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Rev Neurol (Paris) 178 : 64-73, 2022   3. Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins AL 3rd, et al : Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery 42 : 352-360; discussion 360-362, 1998   4. Chen L, Pandey S, Shen R, Xu Y, Zhang Q : Increased systemic immuneinflammation index is associated with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage patients. Front Neurol 12 : 745175, 2021    5. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al : Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43 : 1711-1737, 2012   6. Dzenko KA, Song L, Ge S, Kuziel WA, Pachter JS : CCR2 expression by brain microvascular endothelial cells is critical for macrophage transendothelial migration in response to CCL2. Microvasc Res 70 : 53-64, 2005   9. Gusdon AM, Savarraj JPJ, Shihabeddin E, Paz A, Assing A, Ko SB, et al : Time course of peripheral leukocytosis and clinical outcomes after aneurysmal subarachnoid hemorrhage. Front Neurol 12 : 694996, 2021    10. Lawton MT, Vates GE : Subarachnoid hemorrhage. N Engl J Med 377 : 257-266, 2017   11. Lee H, Perry JJ, English SW, Alkherayf F, Joseph J, Nobile S, et al : Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Neurosurg 130 : 1914-1921, 2018  12. Ma C, Zhou W, Yan Z, Qu M, Bu X : Toll-like receptor 4 (TLR4) is associated with cerebral vasospasm and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 55 : 878-884, 2015    13. Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al : Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir Suppl 115 : 27-31, 2013   14. Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al : Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 43 : 1463-1469, 2012   15. Maruhashi T, Higashi Y : An overview of pharmacotherapy for cerebral vasospasm and delayed cerebral ischemia after subarachnoid hemorrhage. Expert Opin Pharmacother 22 : 1601-1614, 2021   16. McMahon CJ, Hopkins S, Vail A, King AT, Smith D, Illingworth KJ, et al : Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg 5 : 512-517, 2013   17. Provencio JJ : Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: a review. Acta Neurochir Suppl 115 : 233-238, 2013    18. Ray B, Tinsley L, Ford L, Thompson DM, Sidorov EV, Bohnstedt BN : Trends of platelet volume index predicts delayed cerebral ischemia after subarachnoid hemorrhage. World Neurosurg 111 : e624-e631, 2018   20. Srinivasan A, Aggarwal A, Gaudihalli S, Mohanty M, Dhandapani M, Singh H, et al : Impact of early leukocytosis and elevated high-sensitivity C-reactive protein on delayed cerebral ischemia and neurologic outcome after subarachnoid hemorrhage. World Neurosurg 90 : 91-95, 2016   21. Suzuki H, Kanamaru H, Kawakita F, Asada R, Fujimoto M, Shiba M : Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol Histopathol 36 : 143-158, 2021  22. Unda SR, Birnbaum J, Labagnara K, Wong M, Vaishnav DP, Altschul DJ : Peripheral monocytosis at admission to predict cerebral infarct and poor functional outcomes in subarachnoid hemorrhage patients. World Neurosurg 138 : e523-e529, 2020   23. Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al : Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41 : 2391-2395, 2010   24. Wang Z, Pei W, Chen L, Ning Y, Luo Y : Mean platelet volume/platelet count ratio is associated with poor clinical outcome after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 29 : 105208, 2020  
|
|


















