Kula, GГјnay, KayabaЕҹ, AktГјrk, Kula, TГјtГјncГјler, SГјt, and Solak: Neutrophil to Lymphocyte Ratio and Serum Biomarkers : A Potential Tool for Prediction of Clinically Relevant Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage
Abstract
Objective
Subarachnoid hemorrhage (SAH) is a condition characterized by bleeding in the subarachnoid space, often resulting from the rupture of a cerebral aneurysm. Delayed cerebral ischemia caused by vasospasm is a significant cause of mortality and morbidity in SAH patients, and inflammatory markers such as systemic inflammatory response index (SIRI), systemic inflammatory index (SII), neutrophil-to-lymphocyte ratio (NLR), and derived NLR (dNLR) have shown potential in predicting clinical vasospasm and outcomes in SAH patients. This article aims to investigate the relationship between inflammatory markers and cerebral vasospasm after aneurysmatic SAH (aSAH) and evaluate the predictive value of various indices, including SIRI, SII, NLR, and dNLR, in predicting clinical vasospasm.
Methods
A retrospective analysis was performed on a cohort of 96 patients who met the inclusion criteria out of a total of 139 patients admitted Trakya University Hospital with a confirmed diagnosis of aSAH between January 2013 and December 2021. Diagnostic procedures, neurological examinations, and laboratory tests were performed to assess the patients' condition. The StudentвҖҷs t-test compared age variables, while the chi-square test compared categorical variables between the non-vasospasm (NVS) and vasospasm (VS) groups. Receiver operating characteristic (ROC) curve analyses were used to evaluate the diagnostic accuracy of laboratory parameters, calculating the area under the ROC curve, cut-off values, sensitivity, and specificity. A significance level of p<0.05 was considered statistically significant.
Results
The study included 96 patients divided into two groups : NVS and VS. Various laboratory parameters, such as NLR, SII, and dNLR, were measured daily for 15 days, and statistically significant differences were found in NLR on 7 days, with specific cut-off values identified for each day. SII showed a significant difference on day 9, while dNLR had significant differences on days 2, 4, and 9. Graphs depicting the values of these markers for each day are provided.
Conclusion
Neuroinflammatory biomarkers, when used alongside radiology and scoring scales, can aid in predicting prognosis, determining severity and treatment decisions for aSAH, and further studies with larger patient groups are needed to gain more insights.
Key Words: Aneurysmal subarachnoid hemorrhage В· Neutrophil-to-lymphocyte ratio В· Systemic immune-inflammation index В· System inflammation response index В· Serum markers В· Cerebral vasospasm.
INTRODUCTION
Subarachnoid hemorrhage (SAH) is an accumulation of blood in the subarachnoid space after the rupture of a cerebral aneurysm. It is associated with high mortality and morbidity rates [ 1, 6]. The annual incidence of aneurysmatic SAH (aSAH) is nine cases per 100000 people [ 6, 15, 16, 18]. The etiology of SAH is divided into traumatic and non-traumatic causes. The latter kind is more common, and 85% of these are caused by intracranial aneurysm rupture. The remaining 15% of cases do not have any vascular lesions. Hypertension (HT), smoking, family history, and history of previous bleeding aneurysms are major risk factors for ruptured intracranial aneurysms [ 21]. The prognosis for SAH is determined by factors such as sex, HT, the presence of an aneurysm or diabetes mellitus (DM), and medication use [ 6, 16, 18]. The mortality rate is between 17% and 50% [ 15, 16, 18]. SAH also causes morbidities that require care or prevent the patient from returning to work [ 18]. The leading cause of mortality and morbidity after aSAH is delayed cerebral ischemia (DCI), mainly caused by vasospasm [ 1]. Vasospasm is a reaction to arterial rupture followed by the narrowing of the vesselвҖҷs lumen [ 5, 7]. It occurs on the third day after a SAH, peaks on approximately the seventh day, and persists for 2 weeks. Its pathophysiology involves the nitric oxide (NO) pathway. NO causes an increase in intracellular calcium (Ca 2+) through the production of cyclic guanosine monophosphate, relaxation of smooth muscle cells, and vasodilation in the main cerebral arteries4). Vasospasm is the most common complication of a SAH and requires urgent treatment [ 5]. It can be diagnosed clinically and angiographically, but the gold standard criterion is cerebral angiography : angiographic vasospasm can be seen in 70% of patients with an aSAH, whereas clinical vasospasm is seen in 20-30% of patients [ 9]. Predictive and prognostic factors for outcomes in patients with SAH are still unclear [ 16]. However, the World Federation of Neurological Surgeons (WFNS) scale, which is calculated based on factors such as motor deficit and the patientвҖҷs Glasgow coma scale (GCS), has prognostic value [ 18]. In addition, inflammatory markers are important indicators in outcome prediction and prognosis [ 1, 19]. The SAH mechanism starts with leukocytosis and platelet activation, stimulating an inflammatory response that causes brain damage and, eventually, vasospasm. At this point, thrombosis is induced, inflammatory molecules are released, neutrophils increase the inflammatory response, damage occurs to the blood-brain barrier, inflammatory mediators are released, and monocytes interact with platelets and endothelial cells, promoting inflammatory and thrombotic pathways. In contrast, lymphocytes play an important role in the anti-inflammatory response [ 19]. System inflammation can be detected by the systemic inflammatory response index (SIRI) and the systemic inflammatory index (SII), which are used in the prognosis of several types of cancer [ 19]. The neutrophil-to-lymphocyte ratio (NLR) indicates inflammation and can be used as a predictor of tumors and cardiovascular disease [ 2]. The NLR primarily reflects inflammatory damage, whereas SII provides overarching information on inf lammation, hemostasis, immunity, and thrombosis [ 14]. Recent studies have found significant associations between increased SIRI, SII, and NLR values and poor cancer prognoses [ 2, 19]. Inflammatory markers involving all these processes may help predict the aSAH prognosis [ 19]. This study investigated the relationship between inflammatory markers and cerebral vasospasm after aSAH and the contribution of SIRI, SII, NLR, and derived NLR (dNLR) indices in predicting clinical vasospasm.
MATERIALS AND METHODS
Ethics
This study was approved by the Trakya University Faculty of Medicine Scientific Research Ethics Committee (TГңTF-GOBAEK 2022/410).
Patient population
A retrospective analysis was performed on 139 patients admitted to Trakya University Hospital for aSAH between January 2013 and December 2021, of which 96 were included in this study. The inclusion criteria were as follows : 1) being diagnosed with aSAH and admitted to the hospital within the first 24 hours from the onset of the complaint; 2) WFNS stage вүӨ2; 3) no neurological deficit at the time of admission; 4) age >18 years; and 5) surgical clipping or endovascular treatment within the first 3 days after admission. Patients with any concomitant infectious disease, autoimmune disease, cancer, or rebleeding as a complication were excluded to ensure the homogeneity of the patient group ( Fig. 1).
Technical analysis
Patients underwent non-contrast cranial computed tomography (CT) imaging of the SAH within the first 24 hours. Most were diagnosed with SAH by CT. A вҖңsuspectвҖқ group (not found to have SAH by CT) was subsequently diagnosed with SAH by lumbar puncture. After detecting a SAH, patients underwent cranial CT angiography (CTA) and/or four-system selective cerebral angiography.
Patients underwent daily neurologic examinations to determine DCI secondary to cerebral vasospasm. Since DCI is considered as a clinical entity, non-contrast cranial CT was performed, and wide laboratory parameters were examined in order to diagnose possible DCI in patients who developed focal deficit and/or decrease in level of consciousness. Potential causes such as infection, seizure, hydrocephalus, rebleeding, hypotension, metabolic disturbances that could explain the neurological deterioration were excluded. Patients with progressive decrease in GCS in consecutive follow-ups were considered as clinical vasospasm.
Digital subtraction angiography (DSA) was performed to confirm possible cerebral vasospasm in patients with neurological deterioration and clinical vasospasm.
Data collection
Patient information was collected from the hospital database. While collecting patient data, we considered the following topics : age, sex, medical history (DM and HT), neurologic status of the patient at admission (WFNS grading scale), Fisher scale, location and shape of the aneurysm, clinical vasospasm, transamine use, and surgical clip/endovascular.
Neurological examinations were performed by two neurosurgeons with 20 and 15 years of experience (B.T. and Y.A., respectively), and cerebral angiography was performed by an interventional neuroradiologist with 15 years of experience (O.K.).
Laboratory parameters
Counts of leukocytes (109/L), platelets (109/L), neutrophils (109/L), lymphocytes (109/L), and monocytes (109/L) were evaluated from standard blood tests obtained daily on admission and for the following 15 days.
SIRI, SII, NLR, and dNLR were calculated using the following formulas : SIRI = neutrophil count Г— monocyte count / lymphocyte count; SII = platelet count Г— neutrophil count / lymphocyte count; NLR = neutrophil count / lymphocyte count; dNLR = (leukocyte count вҲ’ neutrophil count) / lymphocyte count.
Statistical analysis
Results are expressed as meansВұstandard deviations or numbers (%). The StudentвҖҷs t-test was used to compare age variables between the non-vasospasm (NVS) and vasospasm (VS) groups. The chi-square test was used to compare categorical variables (sex, past medical history, aneurysmal location, aneurysm morphology after rupture, clinical scores, tranexamic acid, and surgical modality) between the NVS and VS groups. The diagnostic accuracy of the laboratory parameters was assessed using receiver operating characteristic (ROC) curve analyses. The area under the ROC curve (AUC) and cut-off values for the laboratory parameters and sensitivity and specificity values were calculated. A result with p<0.05 was considered statistically significant.
RESULTS
This study included 96 patients, of whom 66 were in the NVS group and 30 were in the VS group. The mean age was 54.6Вұ13.6 years in the NVS group and 54.9Вұ12.1 years in the VS group ( p=0.893). The numbers of males and females were 34 and 32 in the NVS group and 13 and 17 in the VS group, respectively. The medical history, aneurysm location and morphology, clinical scoring, tranexamic acid administration, and surgical modality variables of patients in the VS and NVS groups are detailed in Table 1. Laboratory values were checked daily for all parameters. Cut-off, sensitivity, and specificity values were calculated on the day of admission and the following 15 days.
Statistically significant differences were found for the NLR on 7 days (6, 7, 8, 9, 10, 12, and 13). Cut-off values for each day were ( Table 2) : day 6, >12.2 (AUC, 0.711; sensitivity, 52.6; specificity, 93.7; p=0.008); day 7, >8.5 (AUC, 0.756; sensitivity, 72.7; specificity, 78.8; p<0.001); day 8, >9.2 (AUC, 0.688; sensitivity, 65.0; specificity, 84.0; p=0.030); day 9, >11.9 (AUC, 0.733; sensitivity, 43.5; specificity, 92.9; p=0.001); day 10, >13.7 (AUC, 0.680; sensitivity, 45.5; specificity, 87.0; p=0.025); day 12, >11.0 (AUC, 0.688; sensitivity, 60.0; specificity, 85.7; p=0.029); and day 13, >7.7 (AUC, 0.785; sensitivity, 75.0; specificity, 76.2; p<0.001). Graphs of the days with significant differences in NLR are shown in Fig. 2. A statistically significant difference was found for the SII only on day 9. The day 9 cut-off value was вүӨ156.1 (AUC, 0.666; sensitivity, 60.9; specificity, 85.7; p=0.048; Table 2). No statistically significant differences were found for the SIRI ( Table 2). Statistically significant differences were found for the dNLR on 3 days (2, 4, and 9). The cut-off values of these days were : day 2, >1.7 (AUC, 0.653; sensitivity, 80.0; specificity, 53.7; p=0.044); day 4, >1.8 (AUC, 0.695; sensitivity, 61.1; specificity, 77.8; p=0.011); and day 9, >1.5 (AUC, 0.704; sensitivity, 87.0; specificity, 50.0; p=0.006).
Fig. 3 shows the values of all markers calculated for each day.
DISCUSSION
In our study, the cut-off values for the NLR were statistically significant in detecting clinical VS, especially on the days with the most intense VS.
The patients included in this study were conscious and cooperative and did not have neurologic deficits at presentation. Therefore, neurologic deterioration was quickly recognized, and clinical VS was confirmed by performing DSA in these patients. In the literature, transcranial Doppler ultrasound (TCD) and CTA are used to evaluate VS. TCD is a method with low sensitivity and specificity because its imaging success in detecting VS is operator-dependent. The appropriate acoustic window cannot be found, meaning additional imaging is required [ 21]. In addition, CTA is more commonly used to visualize patients in the acute phase. CTA has higher sensitivity and is more helpful in predicting DCI [ 7, 21]. However, the inability to perform an optimal neurologic examination in unconscious patients causes problems, such as not knowing the day CTA or DSA should be performed in this patient group and the impossibility of performing these methods daily. Therefore, published studies on VS are not very useful in daily practice because they do not specifically evaluate VS clinically, and angiographic VS is seen in more than half of this patient group. However, our study is the first in its field to use inflammatory markers and indices for clinical VS prediction and clinical VS diagnosis is supported by DSA, and it supports the diagnosis with more precise results. As a result of current studies, neuroinflammation, which is involved in aSAH development, has also been suggested as an indicator of its clinical consequences and complications. Therefore, many studies have focused on inflammatory markers such as NLR, SIRI, or SII in the prognosis and prediction of aSAH [ 16]. The mentioned markers are cost-effective, not easily affected by many factors, sensitive and reliable in detecting most clinical changes, and easy to calculate. These advantages increase their usability in the treatment decision and process [ 16, 17]. Feghali et al. [ 9] showed that the mean NLR value was higher in patients who developed VS, although the difference was not statistically significant (10.49 > 9.60). Giede-Jeppe et al. [ 11] performed a ROC analysis to differentiate unfavorable and favorable outcome prediction in patients with aSAH, determining a cut-off value for the NLR of 7.05. Its sensitivity was 74.5% and its specificity was 69.3%. Dowlati et al. [ 8] calculated the cut-off value as 8. Guo et al. [ 12] found the cut-off value for the NLR to be 12.03, while the AUC was 0.805 ( p<0.001) in the ROC analysis. In the literature, ROC analysis has been calculated only for specific days or the moment of application, whereas in our study, values for each day were calculated for 15 days. In our study, the highest sensitivity for the NLR (75.0%) was on day 13, while the highest specificity was on day 6 (93.7%)вҖ”a high specificity value compared to the literature. In addition to the above biomarkers, looking at the dNLR values is also valuable. We found that there was a significant difference on 3 days. However, we could not find a detailed study on this marker in the literature review.
While many previous studies have supported the NLR value in the prognosis of aSAH, others found the SIRI and SII values to be stronger prognostic factors. Yun et al. [ 19] found the cut-off value for the SIRI to be 3.2 ( p<0.001), while Zhang et al. [ 20] found the cut-off value to be 4 (AUC, 0.671; sensitivity, 66.0%; specificity, 69.5%). In our study, no statistically significant difference was found for the SIRI. The good neurologic status of our patient group, and the fact that we worked with a smaller patient group to evaluate clinical VS, may have contributed to the lack of statistically significant differences in our study compared to those generally seen in the literature. Chen et al. [ 3] found the cut-off value of the SII to be 1.424, while Geraghty et al. [ 10] calculated it to be 1.924 in patients with VS. Luo et al. [ 14] found the cut-off value to be 2344.65 (sensitivity, 66.7%; specificity, 75.0%; AUC, 0.692; p<0.05). In our study, a significant difference was found only on day 9, where the cut-off value was вүӨ156.1 (sensitivity, 60.9; specificity, 85.7; AUC, 0.666; p=0.048). In the study by Geraghty et al. [ 10], the patients who developed VS were younger than those who did not. In the same study, the male sex ratio was higher in patients with VS [ 10]. In the study by Feghali et al. [ 9], 85% of aneurysms were evaluated to be located in the anterior circulation. Geraghty et al. [ 10] also found this location more frequently in those who developed VS. In the current literature, the relationship between comorbidities, especially DM and VS, has not been fully considered. While elevated blood glucose levels at hospital admission in patients with aSAH indicate its severe condition, it is not considered a predictor of VS [ 13]. In the study by Geraghty et al. [ 10], the proportion of patients with DM was similar in the group with and without VS. While no significant difference was detected in the same study, patients with HT were more common in the group that did not develop VS [ 10]. In the study of Geraghty et al. [ 10], patients with a high Modified Fisher score (grades 3 and 4) were more likely to be in the group with VS, while those with a low score were more likely to be in the group without VS. Those with higher GCS scores were mostly in the NVS group [ 10]. Our study found that the NVS group tended to be grade 2, and the VS group tended to be grade 3. In our study, we found that certain clinical variables, including sex, age, aneurysm location, DM, HT, and various clinical and radiologic scoring systems, did not show statistically significant differences between the NVS and VS groups. This observation may be attributed to the specific patient population we selected, which mainly comprised conscious and cooperative individuals with good GCS and WFNS scores at presentation (grades 1 and 2). As we aimed to specifically evaluate clinical VS in this patient group, the lack of significant differences in these variables should be interpreted in light of the selected patient cohort.
Since our study was retrospective, we could not accurately assess how many days the biomarkers were significant before VS because we did not know exactly on which day clinical VS developed in some patients. In addition, systemic bias, a small sample size, and a single-center study design indicate the need for further studies. However, in most published studies, only the values at the time of hospital admission were considered. In contrast, separate cut-off values were obtained daily over 15 days in our study. In addition, this is the first study to use biomarkers and indices to evaluate clinical VS specifically.
CONCLUSION
With neuroinflammatory biomarkers, our knowledge of the innate immune components involved in the pathogenesis of aSAH can help predict prognosis and treatment decisions. These are not biomarkers that can be used alone. However, combined with parameters such as radiology or scoring scales, they may help determine severity and prognosis at admission, more active patient follow-up, and more precise treatment. Further studies with larger patient groups will shed more light on this issue. Instead of costly and labor-intensive tests, markers calculated from blood samples that are routinely performed and cost-effective encourage these studies.
Fig.В 1.
Study flowchart. aSAH : aneurysmatic subarachnoid hemorrhage, WFNS : World Federation of Neurological Surgeons. 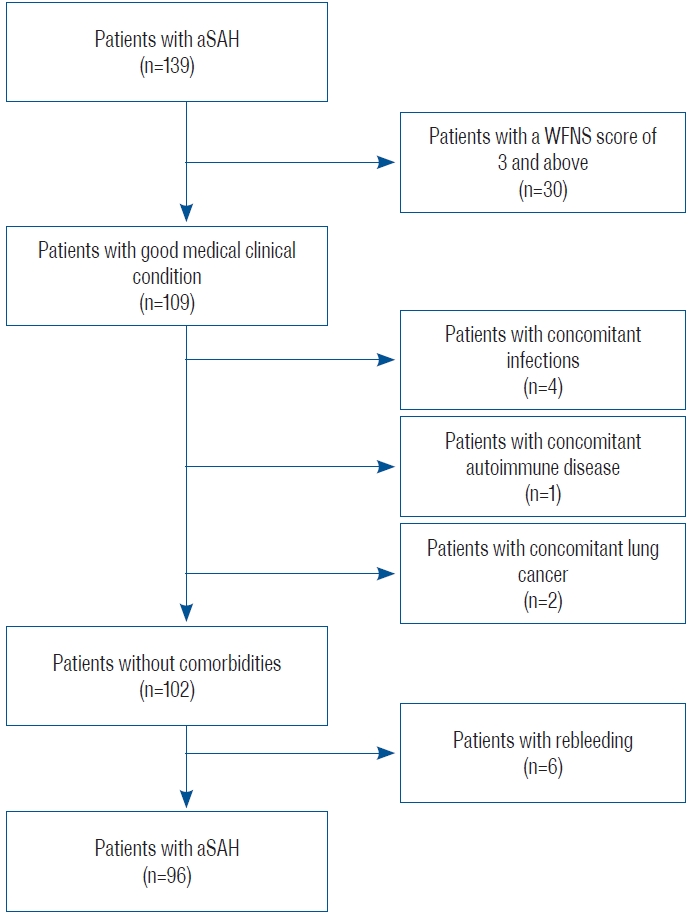
Fig.В 2.
Temporal profiles of systemic inflammatory response index (SIRI), systemic inflammatory index (SII), neutrophil-to-lymphocyte ratio (NLR), and derived NLR (dNLR) plotted during fifteen days following subarachnoid hemorrhage. 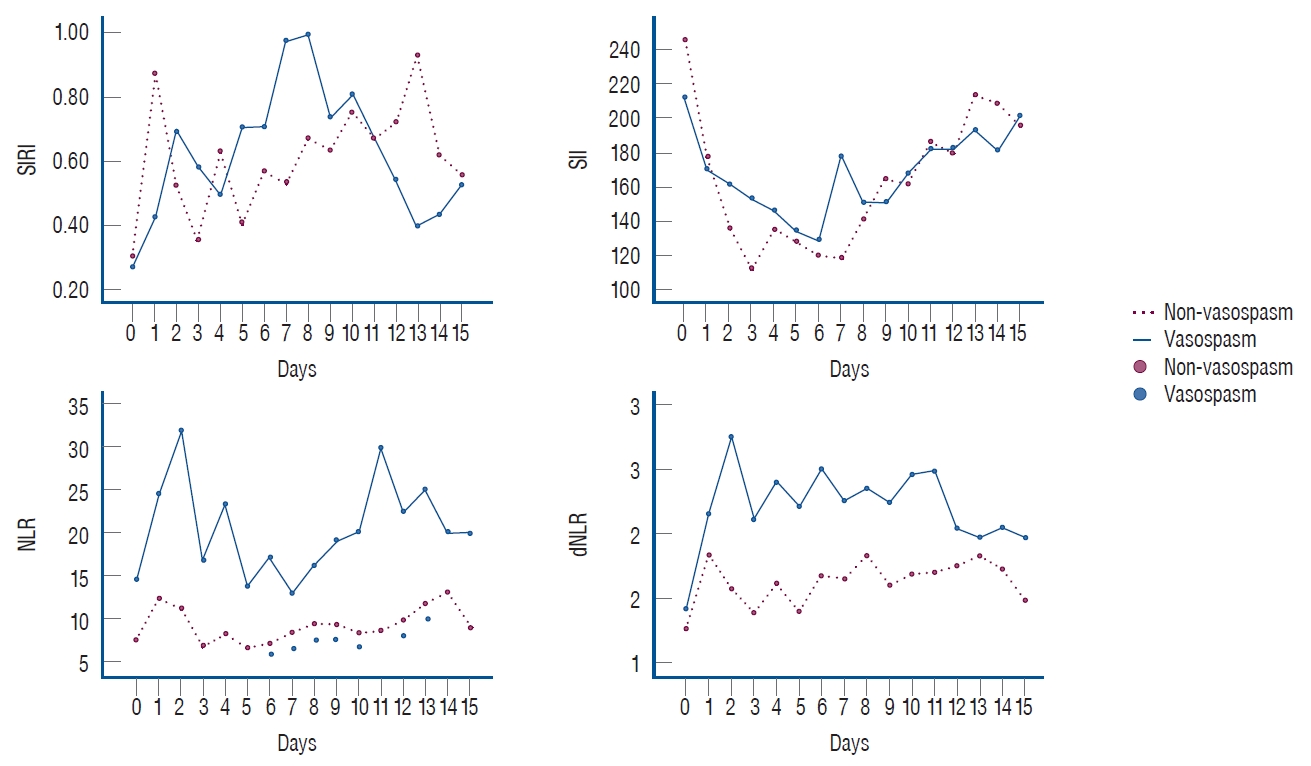
Fig.В 3.
Receiver operating characteristic curve analysis in statistically significant days of NLR values. NLR : neutrophil-to-lymphocyte ratio. 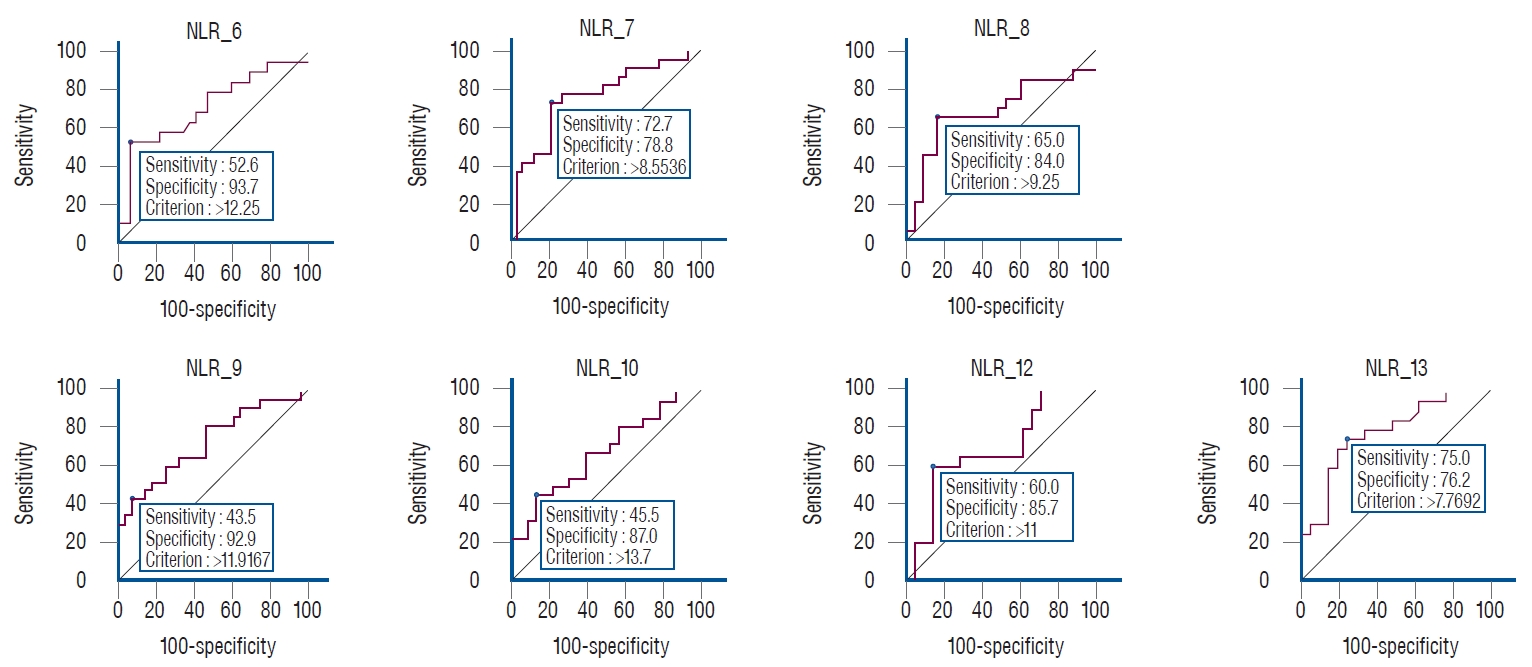
TableВ 1.
Descriptive statistics of aSAH patients
|
NVS (n=66) |
VS (n=30) |
p-value |
|
Gender |
|
|
0.601 |
|
вҖғFemale (n=47) |
34 (72.3) |
13 (27.7) |
|
|
вҖғMale (n=49) |
32 (65.3) |
17 (34.7) |
|
|
Past medical history |
|
|
|
|
вҖғDM |
|
|
1.000 |
|
вҖғвҖғ- |
59 (68.6) |
27 (31.4) |
|
|
вҖғвҖғ+ |
7 (70.0) |
3 (30.0) |
|
|
вҖғHT |
|
|
1.000 |
|
вҖғвҖғ- |
41 (69.5) |
18 (30.5) |
|
|
вҖғвҖғ+ |
25 (67.6) |
12 (32.4) |
|
|
Aneurysmal location |
|
|
0.076 |
|
вҖғACA |
3 (33.3) |
6 (66.7) |
|
|
вҖғMCA |
19 (65.5) |
10 (34.5) |
|
|
вҖғICA |
10 (90.9) |
1 (9.1) |
|
|
вҖғVB |
3 (50.0) |
3 (50.0) |
|
|
вҖғACoA |
21 (77.8) |
6 (22.2) |
|
|
вҖғPCoA |
10 (71.4) |
4 (28.6) |
|
|
Aneurysm morphology after rupture |
|
|
0.440 |
|
вҖғSaccular (regular shape) |
9 (60.0) |
6 (40.0) |
|
|
вҖғSaccular (irregular shape) |
55 (70.5) |
23 (29.5) |
|
|
вҖғFusiform |
1 (100.0) |
0 (0.0) |
|
|
вҖғMycotic |
0 (0.0) |
1 (100.0) |
|
|
вҖғDissecting |
1 (100.0) |
0 (0.0) |
|
|
Clinical scores |
|
|
|
|
вҖғWFNS |
|
|
0.087 |
|
вҖғвҖғ1 |
54 (74.0) |
19 (26.0) |
|
|
вҖғвҖғ2 |
12 (52.2) |
11 (47.8) |
|
|
вҖғModified Fisher scale |
|
|
0.076 |
|
вҖғвҖғ0 |
1 (33.3) |
2 (66.7) |
|
|
вҖғвҖғ1 |
5 (62.5) |
3 (37.5) |
|
|
вҖғвҖғ2 |
34 (82.9) |
7 (17.1) |
|
|
вҖғвҖғ3 |
16 (59.2) |
11 (40.8) |
|
|
вҖғвҖғ4 |
10 (58.9) |
7 (41.1) |
|
|
Tranexamic acid |
|
|
1.000 |
|
вҖғ- |
36 (67.9) |
17 (32.1) |
|
|
вҖғ+ |
30 (69.8) |
13 (30.2) |
|
|
Surgical modality |
|
|
0.510 |
|
вҖғSurgical clipping |
47 (66.2) |
24 (33.8) |
|
|
вҖғEndovascular embolization |
19 (76.0) |
6 (24.0) |
|
TableВ 2.
ROC analysis of laboratory parameters by days
|
Day 0 |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
Day 8 |
Day 9 |
Day 10 |
Day 11 |
Day 12 |
Day 13 |
Day 14 |
Day 15 |
|
SIRI |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AUC |
0.576 |
0.540 |
0.552 |
0.516 |
0.508 |
0.543 |
0.592 |
0.555 |
0.532 |
0.540 |
0.545 |
0.581 |
0.502 |
0.526 |
0.536 |
0.529 |
|
SE |
0.060 |
0.077 |
0.077 |
0.075 |
0.090 |
0.087 |
0.086 |
0.085 |
0.092 |
0.085 |
0.088 |
0.091 |
0.097 |
0.095 |
0.094 |
0.113 |
|
p-value |
0.210 |
0.602 |
0.499 |
0.832 |
0.931 |
0.621 |
0.287 |
0.516 |
0.730 |
0.637 |
0.607 |
0.377 |
0.980 |
0.784 |
0.700 |
0.798 |
|
Cut-off |
вүӨ0.423 |
>0.444 |
>0.746 |
>0.482 |
>0.514 |
>0.538 |
>0.616 |
>0.975 |
вүӨ0.411 |
>0.940 |
>0.820 |
>1.052 |
вүӨ1.03 |
вүӨ1.02 |
вүӨ1.13 |
вүӨ0.59 |
|
Sensitivity |
70.0 |
53.3 |
65.0 |
78.9 |
55.6 |
70.0 |
68.4 |
40.9 |
25.0 |
47.8 |
50.0 |
38.1 |
70.0 |
70.0 |
85.0 |
53.3 |
|
Specificity |
56.1 |
61.7 |
53.7 |
39.0 |
25.0 |
44.8 |
62.5 |
78.8 |
97.6 |
73.8 |
67.2 |
85.0 |
4.8 |
9.5 |
30.0 |
73.3 |
|
NLR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AUC |
0.537 |
0.611 |
0.591 |
0.592 |
0.648 |
0.559 |
0.711 |
0.756 |
0.688 |
0.733 |
0.680 |
0.629 |
0.688 |
0.785 |
0.511 |
0.591 |
|
SE |
0.072 |
0.075 |
0.087 |
0.087 |
0.085 |
0.088 |
0.080 |
0.069 |
0.087 |
0.072 |
0.080 |
0.089 |
0.086 |
0.072 |
0.097 |
0.108 |
|
p-value |
0.614 |
0.144 |
0.293 |
0.290 |
0.081 |
0.505 |
0.008*
|
<0.001*
|
0.030*
|
0.001*
|
0.025*
|
0.150 |
0.029*
|
<0.001*
|
0.914 |
0.397 |
|
Cut-off |
>14.4 |
>7.6 |
>19.9 |
>10.2 |
>8.0 |
>10.0 |
>12.2 |
>8.5 |
>9.2 |
>11.9 |
>13.7 |
>12.8 |
>11.0 |
>7.7 |
вүӨ13.3 |
>4.4 |
|
Sensitivity |
36.7 |
95.0 |
30.0 |
73.7 |
72.2 |
45.0 |
52.6 |
72.7 |
65.0 |
43.5 |
45.5 |
38.1 |
60.0 |
75.0 |
80.0 |
80.0 |
|
Specificity |
83.3 |
27.7 |
97.6 |
56.1 |
61.1 |
72.4 |
93.7 |
78.8 |
84.0 |
92.9 |
87.0 |
90.0 |
85.7 |
76.2 |
42.1 |
40.0 |
|
SII |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AUC |
0.531 |
0.518 |
0.615 |
0.534 |
0.519 |
0.543 |
0.549 |
0.577 |
0.628 |
0.666 |
0.571 |
0.660 |
0.598 |
0.581 |
0.537 |
0.644 |
|
SE |
0.067 |
0.073 |
0.077 |
0.079 |
0.093 |
0.090 |
0.093 |
0.087 |
0.094 |
0.084 |
0.090 |
0.095 |
0.096 |
0.094 |
0.094 |
0.117 |
|
p-value |
0.647 |
0.806 |
0.140 |
0.669 |
0.843 |
0.634 |
0.596 |
0.376 |
0.175 |
0.048*
|
0.431 |
0.093 |
0.312 |
0.392 |
0.690 |
0.216 |
|
Cut-off |
>235.2 |
>172.9 |
>209.7 |
>136.4 |
вүӨ128.5 |
вүӨ109.2 |
вүӨ133.0 |
вүӨ137.2 |
вүӨ151.8 |
вүӨ156.1 |
вүӨ146.2 |
вүӨ158.1 |
вүӨ147.9 |
вүӨ153.2 |
вүӨ74.1 |
>216.5 |
|
Sensitivity |
30.0 |
80.0 |
40.0 |
84.2 |
38.9 |
30.0 |
47.4 |
54.5 |
55.0 |
60.9 |
54.5 |
57.1 |
40.0 |
45.0 |
20.0 |
66.7 |
|
Specificity |
86.4 |
38.3 |
85.4 |
31.7 |
80.6 |
96.6 |
84.4 |
81.8 |
84.0 |
85.7 |
73.9 |
95.0 |
95.2 |
81.0 |
95.0 |
80.0 |
|
dNLR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AUC |
0.521 |
0.633 |
0.653 |
0.623 |
0.695 |
0.602 |
0.646 |
0.627 |
0.617 |
0.704 |
0.632 |
0.633 |
0.623 |
0.624 |
0.501 |
0.600 |
|
SE |
0.068 |
0.079 |
0.076 |
0.081 |
0.077 |
0.087 |
0.094 |
0.083 |
0.091 |
0.075 |
0.084 |
0.089 |
0.090 |
0.092 |
0.097 |
0.111 |
|
p-value |
0.755 |
0.092 |
0.044*
|
0.129 |
0.011*
|
0.244 |
0.122 |
0.128 |
0.202 |
0.006*
|
0.117 |
0.134 |
0.177 |
0.178 |
0.989 |
0.367 |
|
Cut-off |
>1.6 |
>1.4 |
>1.7 |
>1.7 |
>1.8 |
>1.7 |
>1.7 |
>2.0 |
>1.9 |
>1.5 |
>2.1 |
>1.7 |
>1.9 |
>1.9 |
>1.4 |
>1.5 |
|
Sensitivity |
33.3 |
70.0 |
80.0 |
63.2 |
61.1 |
65.0 |
57.9 |
31.8 |
45.0 |
87.0 |
36.4 |
52.4 |
35.0 |
45.0 |
75.0 |
80.0 |
|
Specificity |
81.8 |
53.2 |
53.7 |
65.9 |
77.8 |
62.1 |
84.4 |
97.0 |
84.0 |
50.0 |
91.3 |
80.0 |
95.2 |
90.5 |
47.4 |
53.3 |
References
1. Bjerkne Wenneberg S, Odenstedt HergГЁs H, Svedin P, Mallard C, Karlsson T, Adiels M, et al : Association between inflammatory response and outcome after subarachnoid haemorrhage. Acta Neurol Scand 143 : 195-205, 2021    2. Cai L, Zeng H, Tan X, Wu X, Qian C, Chen G : The role of the blood neutrophil-to-lymphocyte ratio in aneurysmal subarachnoid hemorrhage. Front Neurol 12 : 671098, 2021    3. Chen L, Pandey S, Shen R, Xu Y, Zhang Q : Increased systemic immuneinflammation index is associated with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage patients. Front Neurol 12 : 745175, 2021    4. Ciurea AV, Palade C, Voinescu D, Nica DA : Subarachnoid hemorrhage and cerebral vasospasm - literature review. J Med Life 6 : 120-125, 2013   5. Clozel M, Watanabe H : BQ-123, a peptidic endothelin ETA receptor antagonist, prevents the early cerebral vasospasm following subarachnoid hemorrhage after intracisternal but not intravenous injection. Life Sci 52 : 825-834, 1993   6. DвҖҷSouza S : Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 27 : 222-240, 2015    7. Djelilovic-Vranic J, Basic-Kes V, Tiric-Campara M, Djozic E, Kulenovic J : Follow-up of vasospasm by transcranial doppler sonography (TCD) in subarachnoid hemorrhage (SAH). Acta Inform Med 25 : 14-18, 2017    8. Dowlati E, Mualem W, Carpenter A, Chang JJ, Felbaum DR, Sur S, et al : Early fevers and elevated neutrophil-to-lymphocyte ratio are associated with repeat endovascular interventions for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 36 : 916-926, 2022    9. Feghali J, Kim J, Gami A, Rapaport S, Caplan JM, McDougall CG, et al : Monocyte-based inflammatory indices predict outcomes following aneurysmal subarachnoid hemorrhage. Neurosurg Rev 44 : 3499-3507, 2021    11. Giede-Jeppe A, Reichl J, SprГјgel MI, LГјcking H, Hoelter P, EyГјpoglu IY, et al : Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg 132 : 400-407, 2019   12. Guo Y, Liu J, Zeng H, Cai L, Wang T, Wu X, et al : Neutrophil to lymphocyte ratio predicting poor outcome after aneurysmal subarachnoid hemorrhage: a retrospective study and updated meta-analysis. Front Immunol 13 : 962760, 2022    14. Luo F, Li Y, Zhao Y, Sun M, He Q, Wen R, et al : Systemic immuneinflammation index predicts the outcome after aneurysmal subarachnoid hemorrhage. Neurosurg Rev 45 : 1607-1615, 2022    15. Marazzi TBM, Mendes PV : Updates on aneurysmal subarachnoid hemorrhage: is there anything really new? Arq Neuropsiquiatr 80( 5 Suppl 1):80-87, 2022    16. Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, et al : Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res 12 : 428-446, 2021    17. NГіbrega Lima Rodrigues de Morais A, Ribeiro BaylГЈo VM, Martins Silva T, Gomes Dos Santos A, Azevedo M, J M de Oliveira A : Is neutrophillymphocyte ratio a useful tool for predicting outcome in subarachnoid hemorrhage? A systematic review. Neurosurg Rev 44 : 3023-3028, 2021    18. Rouanet C, Silva GS : Aneurysmal subarachnoid hemorrhage: current concepts and updates. Arq Neuropsiquiatr 77 : 806-814, 2019   19. Yun S, Yi HJ, Lee DH, Sung JH : Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 30 : 105861, 2021   20. Zhang P, Li Y, Zhang H, Wang X, Dong L, Yan Z, et al : Prognostic value of the systemic inflammation response index in patients with aneurismal subarachnoid hemorrhage and a Nomogram model construction. Br J Neurosurg 2020, [Epub ahead of print] 
|
|



















