Lee, Ahn, and Ryu: Clinical Outcomes Associated with Degree of Hypernatremia in Neurocritically Ill Patients
Abstract
Objective
Hypernatremia is a common complication encountered during the treatment of neurocritically ill patients. However, it is unclear whether clinical outcomes correlate with the severity of hypernatremia in such patients. Therefore, we investigated the impact of hypernatremia on mortality of these patients, depending on the degree of hypernatremia.
Methods
Among neurosurgical patients admitted to the intensive care unit (ICU) in a tertiary hospital from January 2013 to December 2019, patients who were hospitalized in the ICU for more than 5 days and whose serum sodium levels were obtained during ICU admission were included. Hypernatremia was defined as the highest serum sodium level exceeding 150 mEq/L observed. We classified the patients into four subgroups according to the severity of hypernatremia and performed propensity score matching analysis.
Results
Among 1146 patients, 353 patients (30.8%) showed hypernatremia. Based on propensity score matching, 290 pairs were included in the analysis. The hypernatremia group had higher rates of in-hospital mortality and 28-day mortality in both overall and matched population (both p<0.001 and p=0.001, respectively). In multivariable analysis of propensity score-matched population, moderate and severe hypernatremia were significantly associated with in-hospital mortality (adjusted odds ratio [OR], 4.58; 95% confidence interval [CI], 2.15-9.75 and adjusted OR, 6.93; 95% CI, 3.46-13.90, respectively) and 28-day mortality (adjusted OR, 3.51; 95% CI, 1.54-7.98 and adjusted OR, 10.60; 95% CI, 5.10-21.90, respectively) compared with the absence of hypernatremia. However, clinical outcomes, including in-hospital mortality and 28-day mortality, were not significantly different between the group without hypernatremia and the group with mild hypernatremia (p=0.720 and p=0.690, respectively). The mortality rates of patients with moderate and severe hypernatremia were significantly higher in both overall and matched population. Interestingly, the mild hypernatremia group of matched population showed the best survival rate.
Conclusion
Moderate and severe hypernatremia were associated with poor clinical outcomes in neurocritically ill patients. However, the prognosis of patients with mild hypernatremia was similar with that of patients without hypernatremia. Therefore, mild hypernatremia may be allowed during treatment of intracranial hypertension using hyperosmolar therapy.
Key Words: Hypernatremia · Neurosurgery · Intensive care units · Mannitol.
INTRODUCTION
Hypernatremia is routinely detected in intensive care units (ICUs) [ 10, 11, 19, 23] and more frequently in the neurosurgical ICU than in the general ICU [ 10]. Hypernatremia may be associated with various complications in critically ill patients [ 10, 11, 17, 25, 27]. Therefore, ICU-acquired hypernatremia is associated with poor clinical outcomes in critically ill patients [ 11, 19, 22, 27]. Hypernatremia is caused by increased sodium intake, loss of free water, or both [ 17]. Under normal conditions, thirst is the primary defense mechanism against the development of hypernatremia [ 17]. However, this mechanism is disrupted in critically ill patients because their consciousness is generally disturbed by sedation or delirium [ 11, 17]. Moreover, neurocritically ill patients are more likely to develop hypernatremia than patients in general ICUs for several reasons, including impaired thirst mechanisms, altered mentality, and hormonal abnormalities resulting from brain injury [ 11, 12]. In addition, hypernatremia may be induced by treating elevated intracranial pressure (ICP) with hyperosmolar therapies, such as mannitol and hypertonic saline, and control of ICP may be correlated with serum sodium concentration [11,12,24. However, it is unclear whether hypernatremia in patients with intracranial hypertension should be corrected even by reducing or discontinuing hyperosmolar agents. A limited number of studies reported that clinical outcomes correlated with the severity of hypernatremia in neurocritically ill patients [ 26]. It is unknown whether hypernatremia itself is associated with poor prognosis, or is a symptom associated with neurocritical illness. Therefore, the objective of this study was to investigate the impact of hypernatremia on mortality of neurocritically ill patients, depending on the degree of hypernatremia. In addition, we evaluated whether hypernatremia per se was associated with poor prognosis when severity and factors other than hypernatremia were controlled by propensity score matching.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Samsung Medical Center (approved number : SMC 2020-09-082). The requirement for informed consent was waived by the Institutional Review Board due to the study’s retrospective nature.
Study population and design
This is a retrospective, single-center, observational study. Patients admitted to the neurosurgical ICU in a tertiary, referral hospital (Samsung Medical Center, Seoul, Korea) from January 2013 to December 2019 were eligible. We included patients 1) who were hospitalized in the ICU for more than 5 days or died within 5 days after ICU admission and 2) whose serum sodium concentrations were obtained during the ICU admission. We excluded patients 1) with hypernatremia (serum sodium >150 mEq/L) on ICU admission, 2) with insufficient medical records, 3) those who were admitted to departments other than neurosurgery, and 4) those who were transferred to other hospitals and with unknown prognoses ( Fig. 1).
Definitions and outcomes
In this study, baseline characteristics of comorbidities, ICU management, and laboratory data were collected retrospectively using Clinical Data Warehouse. Our center constructed the “Clinical Data Warehouse Darwin-C” designed for investigators to search and retrieve de-identified medical records from the electronic archives. It contains data pertaining to more than 4 million patients. The clinical and laboratory data were extracted from the Clinical Data Warehouse Darwin-C after finalizing the patient list in this study. The levels of serum sodium were measured at least once every morning in all patients. Additional laboratory tests were performed if patients underwent hyperosmolar therapy or when attending physicians or neurosurgeons needed additional tests throughout the day. Hypernatremia was defined as the highest serum sodium level exceeding 150 mEq/L during ICU stay [ 26]. We also classified the patients to determine the association between clinical outcomes and severity of hypernatremia. Patients were divided into four subgroups : no hypernatremia (≤150 mEq/L), mild hypernatremia (151-155 mEq/L), moderate hypernatremia (156-160 mEq/L), and severe hypernatremia (>160 mEq/L) [ 26]. Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated with worst values recorded during the initial 24 hours in the ICU admission [ 6, 14]. If the patient was intubated, the verbal score of Glasgow coma scale (GCS) was estimated using the eye and motor scores as reported previously [ 21]. The primary endpoint was in-hospital mortality and the secondary outcome was 28-day mortality.
Statistical analyses
All data are presented as means±standard deviations for continuous variables and frequencies and proportions for categorical variables. Data were compared using Student’s t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables.
Propensity score matching was used to control the selection bias and the confounding detected in this observational study. Each patient with hypernatremia was matched to one of the control patients with the nearest neighbor matching within calipers determined by the propensity score. A caliper width of 0.2 of the standard deviation of the logit of the propensity score was used for the matching [ 3]. To determine the effectiveness of propensity score matching in controlling the differences between patients with and without hypernatremia, the standardized mean differences (SMDs) were calculated for each variable before and after matching. SMDs less than 10% were considered successful propensity scores matching and balancing the groups. To conduct doubly robust estimation to improve causal inference, we combined the propensity score matching and regression methods. The propensity score-matched population was subjected to multiple logistic regression analysis with stepwise variable selection. The variables included in the propensity score estimation and the other multivariable analyses were age, sex, comorbidities, cause of ICU admission, utilization of organ support modalities, including mechanical ventilators, continuous renal replacement therapy and vasopressors, invasive ICP monitoring devices, hyperosmolar therapy, GCS, and APACHE II score on ICU admission. The cumulative incidences of mortality were calculated by Kaplan-Meier estimates and compared using a log-rank test. All tests were two-sided and p-values less than 0.05 were considered statistically significant. Statistical analyses were performed with R Statistical Software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
A total of 12743 patients were admitted to the neurosurgical ICU during the study period and 1146 patients were included in the analysis. In the study population, 353 patients (30.8%) had hypernatremia ( Fig. 1). Mean age of all patients was 50.0± 22.9 years. The study included 597 male patients (52.1%). Malignancy (56.4%) and hypertension (34.1%) were the most common comorbidities. Brain tumors (39.1%) and intracerebral hemorrhage (18.2%) were the most common reasons for ICU admission. Age and APACHE II score on ICU admission were higher in patients with hypernatremia compared with those without hypernatremia (both p<0.001). However, malignancy was more common in the group with hypernatremia than in the group without ( p<0.001). Vasopressors and mechanical ventilators were more commonly used in patients with hypernatremia compared with those without hypernatremia (both p<0.001). Although the frequency of mannitol use was similar between the two groups ( p=0.293), glycerin was more frequently used in the hypernatremia group ( p<0.001). The mean value of maximum serum sodium level was higher in the hypernatremia group than in the group without hypernatremia (160.2±8.6 vs. 141.9±5.8 mEq/L, p<0.001). The mean value of minimum serum sodium level was also higher in the hypernatremia group than in the nonhypernatremia group (144.3±12.9 vs. 135.1±5.8 mEq/L, p<0.001). Compared with neurocritically ill patients without hypernatremia, those with hypernatremia had increased blood urea nitrogen, glucose, and osmolality, and decreased glomerular filtration rate ( Table 1). However, there were no significant differences in the length of stay in the ICU and hospital between the two groups ( p=0.970 and p=0.232, respectively). After propensity score matching, 290 pairs of data were generated by 1 : 1 individual matching without replacement. No significant imbalance was found in the baseline characteristics between the matched data pairs ( Table 1).
Clinical outcomes
In the overall study population, the rates of in-hospital mortality and 28-day mortality were higher in the hypernatremia group compared with the non-hypernatremia group (45.6% vs. 15.5% and 43.6% vs. 13.5%, both p<0.001) ( Table 1). Clinical outcomes in the propensity score-matched population were similar with those of the entire population. In the propensity score-matched population, the rates of in-hospital mortality and 28-day mortality were also higher in the hypernatremia group compared with the non-hypernatremia group (40.3% vs. 26.9% and 37.9% vs. 24.8%, both p=0.001) ( Table 1). In multivariable analysis of propensity score-matched population, the clinical outcomes, including in-hospital mortality and 28-day mortality, were not significantly different between non-hypernatremia and mild hypernatremia groups ( p=0.720 and p=0.690, respectively) ( Table 2). However, moderate and severe hypernatremia were significantly associated with inhospital mortality (adjusted odds ratio [OR], 4.58; 95% confidence interval [CI], 2.15-9.75 and adjusted OR, 6.93; 95% CI, 3.46-13.90, respectively) and 28-day mortality (adjusted OR, 3.51; 95% CI, 1.54-7.98 and adjusted OR, 10.60; 95% CI, 5.10- 21.90, respectively) compared with the absence of hypernatremia ( Table 2). Finally, multivariable logistic regression analysis revealed that moderate and severe hypernatremia, use of vasopressors (adjusted OR, 3.07; 95% CI, 1.68-5.61), continuous renal replacement therapy (adjusted OR, 5.49; 95% CI, 1.80- 16.70), and GCS on ICU admission (adjusted OR, 0.63; 95% CI, 0.59-0.68) were associated with in-hospital mortality ( Table 2). In survival analysis, the mortality rates of patients with moderate and severe hypernatremia were significantly higher compared with those with mild hypernatremia and without hypernatremia in the overall population and propensity scorematched population ( Fig. 2). Especially, the mild hypernatremia group of matched population showed the best survival rate in the Kaplan-Meier curve. However, the mortality rate of patients with mild hypernatremia was not significantly lower compared with those without hypernatremia (16.1% vs. 27.6%, log-rank test, p=0.163).
Discussion
In this study, we investigated the severity and impact of hypernatremia on mortality of neurocritically ill patients, depending on the degree of hypernatremia. Major findings of this study were as follows. First, poor clinical outcomes were more common in patients with hypernatremia compared with those without hypernatremia in overall and propensity scorematched population. Second, patients with mild hypernatremia in the matched population showed the best survival rate. Finally, multivariable analysis revealed that moderate and severe hypernatremia, use of vasopressors and GCS on ICU admission were significantly associated with in-hospital mortality and 28-day mortality in neurocritically ill patients. Specifically, mild hypernatremia was not associated with poor clinical outcomes in this study.
In neurocritically ill patients, hyperosmolar therapy, including mannitol, glycerin or hypertonic saline, are frequently used to control intracranial hypertension [ 12, 28]. Hypernatremia may occur due to the use of these agents [ 11, 12, 24, 28]. Mild hypernatremia is the goal of serum sodium when hypertonic saline is used to lower ICP continuously [ 28]. Neurocritically ill patients are frequently subjected to hyperosmolar therapy. Hyperosmolar therapy and associated acute kidney injury can aggravate hypernatremia [ 1, 16, 26]. In addition, hypothalamic dysfunction due to brain injury can contribute to hypernatremia [ 20, 26]. Therefore, hypernatremia occurs easily in patients with severe neurological disease compared with those manifesting benign disease. Eventually, it is not easy to determine whether hypernatremia itself is associated with a poor prognosis, or patients with hypernatremia show poor prognosis because of their neurocritical illness. Therefore, a propensity score matching method was used to adjust for this confounder in this study. In brief, moderate and severe hypernatremia were significantly associated with poor clinical outcomes in neurocritically ill patients. The prevalence of ICU-acquired hypernatremia was about 5.7% to 50.7% in previous studies [ 10, 19, 23]. Hypernatremia and its associated hyperosmolar conditions lead to metabolic derangement and organ dysfunction, including abnormal hepatic gluconeogenesis, decreased lactate clearance, increased insulin resistance of peripheral tissues, cardiac dysfunction, muscle cramps and rhabdomyolysis [ 10, 17, 19]. Therefore, hypernatremia itself may be associated with multiple complications, prolonged ICU stay, or even death [ 8, 18, 19, 22, 26, 27]. In addition, hypernatremia leads to increased cellular dehydration and decreased cerebral edema, which are often the therapeutic goals in neurocritical care. However, these homeostatic changes can injure myelin and even cause neuronal death. Thus, hypernatremia leads to additional secondary brain injury [ 4, 26]. Eventually, hypernatremia is also associated with poor clinical prognosis in neurocritically ill patients [ 5, 10, 11, 15, 16, 20, 26]. In our previous study, hyperosmolality may be associated with acute kidney injury and poor clinical outcomes in neurocritically ill patients [ 7]. The renal toxicity may be aggravated by dehydration or preexisting renal impairment [ 2] and extreme hyperosmolality may cause acute kidney injury [ 13]. Serum osmolality is decided by serum sodium, blood urea nitrogen and glucose and these three are related to osmotic pressure. Increased serum osmolality as result of increased serum levels of sodium, blood urea nitrogen and glucose may be associated with poor clinical outcomes in neurocritically ill patients. Therefore, it is necessary to monitor electrolytes, blood urea nitrogen, creatine, serum osmolality, and osmolar gap while using hyperosmolar agents, including mannitol, to prevent acute kidney injury and other side effects of hyperosmolar therapy [ 9]. In this study, neurocritically ill patients with mild hypernatremia did not manifest worse outcomes compared to those without hypernatremia. However, patients with moderate or severe hypernatremia had worse prognosis than those without hypernatremia. In neurocritically ill patients with mild hypernatremia, favorable clinical outcomes may be associated with the ICP-lowering effect induced by mild hypernatremia with fewer complications than those detected during moderate and severe hypernatremia. Therefore, hyperosmolar agents may not be reduced or discontinued to maintain normal level of serum sodium in case of mild hypernatremia during treatment of patients with intracranial hypertension.
This study has several limitations. First, this was a retrospective review of medical records and the data extracted from Clinical Data Warehouse. The nonrandomized nature of registry data may have resulted in selection bias. Laboratory studies, including serum sodium levels, were not protocolbased. Second, hypernatremia was easily induced with hypertonic saline. Although, a small number of patients used hypertonic saline in this study, they could not be identified from Clinical Data Warehouse due to technical challenges. Third, the distribution of neurosurgical diseases differed from that of the general neurosurgical ICU, and the proportion of patients with brain tumors was particularly high. Although this study still provides valuable insight, prospective large-scale studies are needed to confirm the safety and effectiveness of mild hypernatremia in neurocritically ill patients to arrive at evidence-based conclusions.
CONCLUSION
In this study, moderate and severe hypernatremia were associated with poor clinical outcomes in neurocritically ill patients. However, prognosis of the patients with mild hypernatremia was similar with those without hypernatremia. Therefore, mild hypernatremia may be allowed during the active management of intracranial hypertension using hyperosmolar therapy. Further, it may not be necessary to reduce or discontinue hyperosmolar agents to control mild hypernatremia.
Acknowledgements
We would like to thank Hye Jung Kim, the nursing director of the neurosurgical intensive care unit, for providing excellent advice and engaging in fruitful discussions. We would also like to thank all nurses of the neurosurgery intensive care unit at Samsung Medical Center.
Fig. 1.
Study flow chart. ICU : intensive care unit, Na : sodium level. 
Fig. 2.
Kaplan-Meier survival analyses of propensity score-mached population (p<0.001). 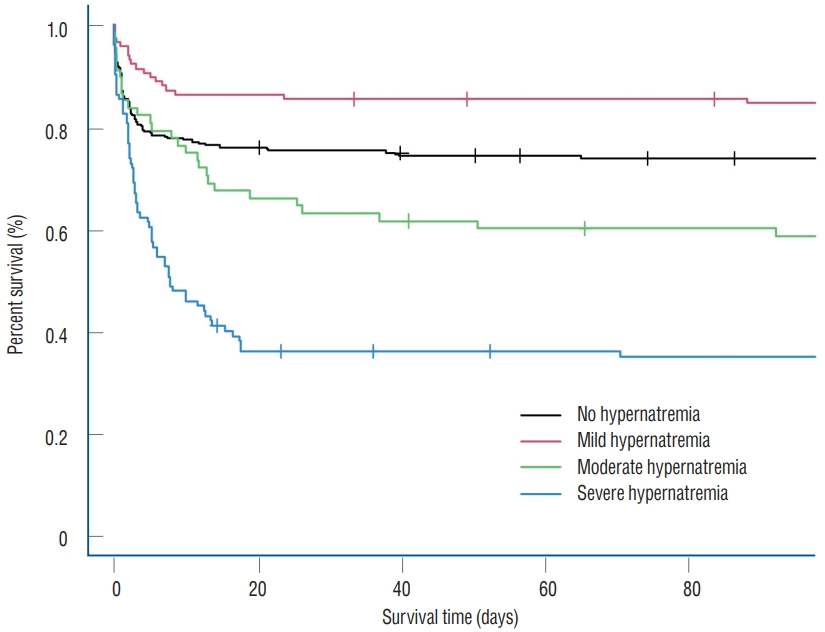
Table 1.
Baseline characteristics of study population
|
Overall study population
|
Propensity score-matched population
|
|
No hypernatremia (n=793) |
Hypernatremia (n=353) |
p-value |
SMD |
No hypernatremia (n=290) |
Hypernatremia (n=290) |
p-value |
SMD |
|
Age (years) |
47.5±23.8 |
55.8±19.6 |
<0.001 |
0.381 |
54.0±19.4 |
54.7±20.0 |
0.678 |
0.035 |
|
Sex, male |
423 (53.3) |
174 (49.3) |
0.229 |
0.081 |
146 (50.3) |
143 (49.3) |
0.868 |
0.021 |
|
Comorbidities |
|
|
|
|
|
|
|
|
|
|
Malignancy |
486 (61.3) |
160 (45.3) |
<0.001 |
0.324 |
137 (47.2) |
143 (49.3) |
0.678 |
0.041 |
|
Hypertension |
258 (32.5) |
133 (37.7) |
0.104 |
0.108 |
103 (35.5) |
103 (35.5) |
0.999 |
<0.001 |
|
Diabetes mellitus |
91 (11.5) |
62 (17.6) |
0.007 |
0.173 |
49 (16.9) |
47 (16.2) |
0.911 |
0.019 |
|
Chronic kidney disease |
56 (7.1) |
24 (6.8) |
0.972 |
0.010 |
23 (7.9) |
20 (6.9) |
0.751 |
0.039 |
|
Cardiovascular disease |
30 (3.8) |
15 (4.2) |
0.833 |
0.024 |
14 (4.8) |
12 (4.1) |
0.841 |
0.033 |
|
Chronic liver disease |
28 (3.5) |
12 (3.4) |
0.999 |
0.007 |
10 (3.4) |
10 (3.4) |
0.999 |
<0.001 |
|
Habitual risk factors |
|
|
|
|
|
|
|
|
|
Current alcohol consumption |
156 (19.7) |
95 (26.9) |
0.008 |
0.172 |
73 (25.2) |
73 (25.2) |
0.999 |
<0.001 |
|
Current smoking |
83 (10.5) |
47 (13.3) |
0.193 |
0.088 |
34 (11.7) |
35 (12.1) |
0.999 |
0.011 |
|
Cause of ICU admission |
|
|
<0.001 |
0.503 |
|
|
0.985 |
0.099 |
|
Brain tumor |
356 (44.9) |
92 (26.1) |
|
|
82 (28.3) |
88 (30.3) |
|
|
|
Intracerebral hemorrhage |
123 (15.5) |
85 (24.1) |
|
|
63 (21.7) |
69 (23.8) |
|
|
|
Traumatic brain injury |
89 (11.2) |
67 (19.0) |
|
|
54 (18.6) |
47 (16.2) |
|
|
|
Subarachnoid hemorrhage |
90 (11.3) |
61 (17.3) |
|
|
42 (14.5) |
43 (14.8) |
|
|
|
Elective vascular surgery |
72 (9.1) |
19 (5.4) |
|
|
19 (6.6) |
17 (5.9) |
|
|
|
Cerebral infarction |
17 (2.1) |
13 (3.7) |
|
|
11 (3.8) |
10 (3.4) |
|
|
|
Central nervous system infection |
16 (2.0) |
3 (0.8) |
|
|
4 (1.4) |
3 (1.0) |
|
|
|
APACHE II score on ICU admission |
6.28±6.2 |
9.39±8.0 |
<0.001 |
0.436 |
7.84±7.3 |
8.40±7.7 |
0.373 |
0.074 |
|
Glasgow coma scale on ICU admission |
13.3±3.2 |
10.5±4.6 |
<0.001 |
0.695 |
11.62±4.3 |
11.39±4.4 |
0.516 |
0.054 |
|
ICU management |
|
|
|
|
|
|
|
|
|
Mechanical ventilation |
448 (56.5) |
291 (82.4) |
<0.001 |
0.587 |
237 (81.7) |
232 (80.0) |
0.673 |
0.044 |
|
Invasive ICP monitoring |
390 (49.2) |
149 (42.2) |
0.034 |
0.140 |
122 (42.1) |
131 (45.2) |
0.503 |
0.063 |
|
Continuous renal replacement therapy |
27 (3.4) |
13 (3.7) |
0.950 |
0.015 |
14 (4.8) |
13 (4.5) |
0.999 |
0.016 |
|
Use of mannitol*
|
347 (43.8) |
142 (40.2) |
0.293 |
0.072 |
116 (40.0) |
113 (39.0) |
0.865 |
0.021 |
|
Use of glycerin*
|
275 (34.7) |
240 (68.0) |
<0.001 |
0.707 |
169 (58.3) |
179 (61.7) |
0.446 |
0.070 |
|
Use of vasopressors |
85 (10.7) |
92 (26.1) |
<0.001 |
0.404 |
59 (20.3) |
65 (22.4) |
0.613 |
0.050 |
|
Laboratory data†
|
|
|
|
|
|
|
|
|
|
Maximal sodium (mEq/L) |
141.9±5.8 |
160.2±8.6 |
<0.001 |
2.488 |
143.2±5.2 |
159.8±8.4 |
<0.001 |
2.395 |
|
Blood urea nitrogen (mg/dL) |
16.0±13.4 |
17.7±13.3 |
0.038 |
0.133 |
17.7±14.8 |
18.2±14.2 |
0.698 |
0.032 |
|
Creatinine (mg/dL) |
0.9±1.2 |
1.0±0.9 |
0.116 |
0.106 |
1.1±1.4 |
1.0±0.9 |
0.304 |
0.086 |
|
Glomerular filtration rate (mL/min/1.73 m2) |
109.3±53.3 |
85.0±39.2 |
<0.001 |
0.520 |
96.5±50.2 |
86.5±41.0 |
0.009 |
0.219 |
|
Maximal glucose (mg/dL) |
204.8±90.8 |
256.6±107.4 |
<0.001 |
0.520 |
228.3±99.5 |
253.4±108.5 |
0.004 |
0.241 |
|
Osmolality (mOsm/kg) |
306.4±15.1 |
340.9±27.4 |
<0.001 |
1.558 |
311.7±16.7 |
340.0±28.5 |
<0.001 |
1.210 |
|
Clinical outcomes†
|
|
|
|
|
|
|
|
|
|
In-hospital mortality |
123 (15.5) |
161 (45.6) |
<0.001 |
|
78 (26.9) |
117 (40.3) |
0.001 |
|
|
28-day mortality |
107 (13.5) |
154 (43.6) |
<0.001 |
|
72 (24.8) |
110 (37.9) |
0.001 |
|
|
ICU length of stay (days) |
11.1±9.7 |
14.4±43.6 |
0.970 |
|
10.5±9.4 |
1.2±47.8 |
0.095 |
|
|
Hospital length of stay (days) |
66.8±240.2 |
53.2±140.6 |
0.232 |
|
71.8±295.9 |
59.6±153.3 |
0.535 |
|
Table 2.
Multivariable analysis of clinical outcomes according to the severity of hypernatremia between propensity score matched population
|
Adjusted odds ratio* (95% CI) |
p-value |
|
In-hospital mortality |
|
|
|
Hypernatremia |
|
|
|
No hypernatremia |
1 |
Reference |
|
Mild hypernatremia |
0.88 (0.43-1.80) |
0.720 |
|
Moderate hypernatremia |
4.58 (2.15-9.75) |
<0.001 |
|
Severe hypernatremia |
6.93 (3.46-13.90) |
<0.001 |
|
Use of vasopressors |
3.07 (1.68-5.61) |
<0.001 |
|
Continuous renal replacement therapy |
5.49 (1.80-16.70) |
0.003 |
|
Glasgow coma scale on admission |
0.63 (0.59-0.68) |
<0.001 |
|
28-day mortality |
|
|
|
Hypernatremia |
|
|
|
No hypernatremia |
1 |
Reference |
|
Mild hypernatremia |
0.86 (0.39-1.86) |
0.690 |
|
Moderate hypernatremia |
3.51 (1.54-7.98) |
0.003 |
|
Severe hypernatremia |
10.60 (5.10-21.90) |
<0.001 |
|
Use of vasopressors |
2.76 (1.47-5.16) |
0.002 |
|
Invasive ICP monitoring |
0.46 (0.26-0.83) |
0.010 |
|
Glasgow coma scale on admission |
0.64 (0.59-0.69) |
<0.001 |
References
1. Aiyagari V, Deibert E, Diringer MN : Hypernatremia in the neurologic intensive care unit: how high is too high? J Crit Care 21 : 163-172, 2006   2. Andrews PM, Cooper M, Verbesey J, Ghasemian S, Rogalsky D, Moody P, et al : Mannitol infusion within 15 min of cross-clamp improves living donor kidney preservation. Transplantation 98 : 893-897, 2014    3. Austin PC : Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10 : 150-161, 2011    4. Ayus JC, Armstrong DL, Arieff AI : Effects of hypernatraemia in the central nervous system and its therapy in rats and rabbits. J Physiol 492 (Pt 1) : 243-255, 1996   5. Beseoglu K, Etminan N, Steiger HJ, Hänggi D : The relation of early hypernatremia with clinical outcome in patients suffering from aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 123 : 164-168, 2014   6. Capuzzo M, Valpondi V, Sgarbi A, Bortolazzi S, Pavoni V, Gilli G, et al : Validation of severity scoring systems SAPS II and APACHE II in a singlecenter population. Intensive Care Med 26 : 1779-1785, 2000    7. Choi HW, Yoon CH, Ryu JA : Acute kidney injury following mannitol infusion in neurosurgical patients. J Neurointensive Care 5 : 9-14, 2022   8. Darmon M, Timsit JF, Francais A, Nguile-Makao M, Adrie C, Cohen Y, et al : Association between hypernatraemia acquired in the ICU and mortality: a cohort study. Nephrol Dial Transplant 25 : 2510-2515, 2010   9. Fink ME : Osmotherapy for intracranial hypertension: mannitol versus hypertonic saline. Continuum (Minneap Minn) 18 : 640-654, 2012  10. Hu B, Han Q, Mengke N, He K, Zhang Y, Nie Z, et al : Prognostic value of ICU-acquired hypernatremia in patients with neurological dysfunction. Medicine (Baltimore) 95 : e3840, 2016    11. Imaizumi T, Nakatochi M, Fujita Y, Nomura R, Watanabe K, Maekawa M, et al : The association between intensive care unit-acquired hypernatraemia and mortality in critically ill patients with cerebrovascular diseases: a single-centre cohort study in Japan. BMJ Open 7 : e016248, 2017    12. Jeon SB, Koh Y, Choi HA, Lee K : Critical care for patients with massive ischemic stroke. J Stroke 16 : 146-160, 2014    13. Kim MY, Park JH, Kang NR, Jang HR, Lee JE, Huh W, et al : Increased risk of acute kidney injury associated with higher infusion rate of mannitol in patients with intracranial hemorrhage. J Neurosurg 120 : 1340-1348, 2014   14. Knaus WA, Draper EA, Wagner DP, Zimmerman JE : APACHE II: a severity of disease classification system. Crit Care Med 13 : 818-829, 1985   16. Li M, Hu YH, Chen G : Hypernatremia severity and the risk of death after traumatic brain injury. Injury 44 : 1213-1218, 2013   17. Lindner G, Funk GC : Hypernatremia in critically ill patients. J Crit Care 28 : 216.e11-20, 2013   18. Lindner G, Funk GC, Lassnigg A, Mouhieddine M, Ahmad SA, Schwarz C, et al : Intensive care-acquired hypernatremia after major cardiothoracic surgery is associated with increased mortality. Intensive Care Med 36 : 1718-1723, 2010    19. Lindner G, Funk GC, Schwarz C, Kneidinger N, Kaider A, Schneeweiss B, et al : Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis 50 : 952-957, 2007   20. Maggiore U, Picetti E, Antonucci E, Parenti E, Regolisti G, Mergoni M, et al : The relation between the incidence of hypernatremia and mortality in patients with severe traumatic brain injury. Crit Care 13 : R110, 2009    21. Meredith W, Rutledge R, Fakhry SM, Emery S, Kromhout-Schiro S : The conundrum of the Glasgow coma scale in intubated patients: a linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma 44 : 839-844; discussion 844-845, 1998  22. O'Donoghue SD, Dulhunty JM, Bandeshe HK, Senthuran S, Gowardman JR : Acquired hypernatraemia is an independent predictor of mortality in critically ill patients. Anaesthesia 64 : 514-520, 2009   23. Polderman KH, Schreuder WO, Strack van Schijndel RJ, Thijs LG : Hypernatremia in the intensive care unit: an indicator of quality of care? Crit Care Med 27 : 1105-1108, 1999  24. Qureshi AI, Suarez JI, Bhardwaj A, Mirski M, Schnitzer MS, Hanley DF, et al : Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: effect on intracranial pressure and lateral displacement of the brain. Crit Care Med 26 : 440-446, 1998  26. Vedantam A, Robertson CS, Gopinath SP : Morbidity and mortality associated with hypernatremia in patients with severe traumatic brain injury. Neurosurg Focus 43 : E2, 2017  27. Waite MD, Fuhrman SA, Badawi O, Zuckerman IH, Franey CS : Intensive care unit-acquired hypernatremia is an independent predictor of increased mortality and length of stay. J Crit Care 28 : 405-412, 2013   28. Wells DL, Swanson JM, Wood GC, Magnotti LJ, Boucher BA, Croce MA, et al : The relationship between serum sodium and intracranial pressure when using hypertonic saline to target mild hypernatremia in patients with head trauma. Crit Care 16 : R193, 2012   
|
|


















