Park: Single Nucleotide Polymorphism in Patients with Moyamoya Disease
Abstract
Moyamoya disease (MMD) is a chronic, progressive, cerebrovascular occlusive disorder that displays various clinical features and results in cerebral infarct or hemorrhagic stroke. Specific genes associated with the disease have not yet been identified, making identification of at-risk patients difficult before clinical manifestation. Familial MMD is not uncommon, with as many as 15% of MMD patients having a family history of the disease, suggesting a genetic etiology. Studies of single nucleotide polymorphisms (SNPs) in MMD have mostly focused on mechanical stress on vessels, endothelium, and the relationship to atherosclerosis. In this review, we discuss SNPs studies targeting the genetic etiology of MMD. Genetic analyses in familial MMD and genome-wide association studies represent promising strategies for elucidating the pathophysiology of this condition. This review also discusses future research directions, not only to offer new insights into the origin of MMD, but also to enhance our understanding of the genetic aspects of MMD. There have been several SNP studies of MMD. Current SNP studies suggest a genetic contribution to MMD, but further reliable and replicable data are needed. A large cohort or family-based design would be important. Modern SNP studies of MMD depend on novel genetic, experimental, and database methods that will hopefully hasten the arrival of a consensus conclusion.
Key Words: Moyamoya disease · Single nucleotide polymorphism · Genetic · Stroke · Cerebrovascular disease.
INTRODUCTION
Moyamoya disease (MMD) is a chronic cerebrovascular occlusive disorder that results in transient ischemia, cerebral infarcts, and hemorrhagic strokes 6,3464,6770). The disease has a bimodal age distribution of peak incidence, with peaks in children who are approximately five years of age and adults in their mid forties 6,1516,3370). MMD occurs higher prevalence in East Asian countries. Further, 15% of MMD cases have a family history of the disease 34). Most juvenile patients develop transient ischemic attacks or cerebral infarctions 12), whereas adult patients are more likely to have a hemorrhagic stroke 33,4551). Although familial occurrence accounts for approximately 9-15% of MMD cases, the majority of cases are sporadic 8,43). This suggests some variant or impairment of genetic sequence in the same disease. Genetic associations with loci on chromosome 3, 6, 8, 10, and 17 and a specific human leukocyte antigen (HLA) haplotype have been reported 14,1722,2352,62), but questions about various genetic penetrations still remain. The current concept of pathogenesis of MMD is more focused on genetic factors rather than on the causes. Possible genetic variants included those of vascular endothelial growth factor, basic fibroblast growth factor, hepatocyte growth factor, transforming growth factor beta 1, granulocyte colony-stimulating factor, platelet-derived growth factor receptor beta, matrix metalloproteinase (MMP), and tissue inhibitor of metalloproteinase-2 38,5972,73). Current single nucleotide polymorphism (SNP) studies suggest a genetic contribution to MMD. Here, we discuss current single nucleotide genetic studies in MMD.
SNP STUDIES
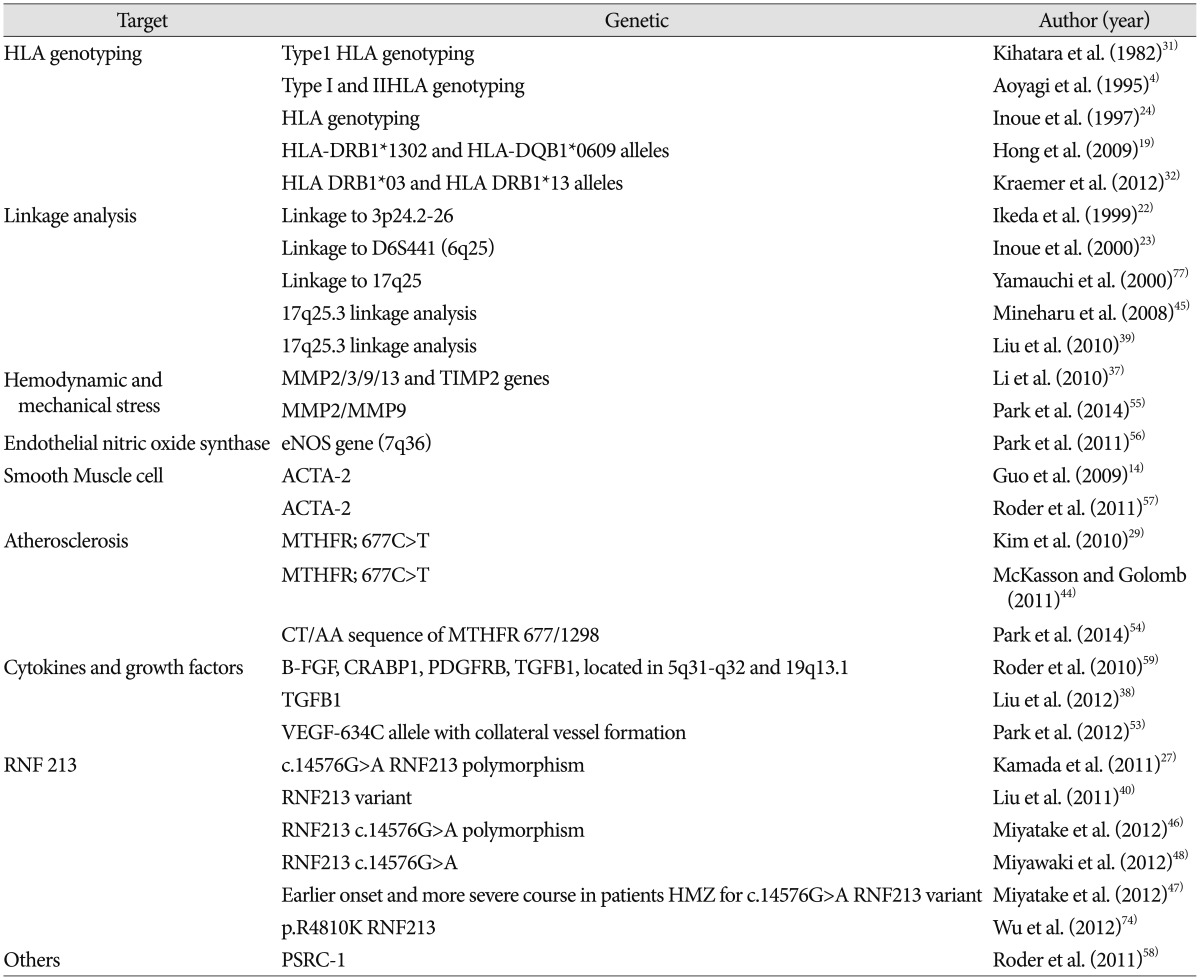
It has been suggested that in families, MMD may be transmitted through a polygenic or autosomal dominant mode with low penetrance 77). Linkage analyses have shown associations with loci 3p24.2-p26 22), 6q25 23), 8q23 62), 10q23.31 14), 12p12 62), and 17q25 45). SNP studies in MMD have mostly focused on mechanical stress on vessels, endothelium, and on the relationship to atherosclerosis ( Table 1).
Association with mechanical stress : angiogenesis and vascular repair genes (TIMP, MMP, Elastin/LIMK1 SNPs)
Dysregulation of tissue inhibitor of metalloproteinases (TIMPs) can disrupt the balance between MMPs and TIMPs, resulting in aberrant vascular smooth muscle cell (SMC) dynamics, ultimately leading to MMD 55). By degrading the neurovascular matrix, MMPs promote blood-brain barrier (BBB) damage, edema, and hemorrhage 5,3642). The balance between MMPs and TIMPs is known to be an important factor of BBB maintenance and vascular angiogenesis 35). Several studies have demonstrated that overexpression of MMP-9 and underexpression of MMP-3, TIMP-1, and TIMP-2 are related to MMD 11). Therefore, any SNPs of proteins involved in this cascade may provoke or protect against ischemic or hemorrhagic MMD. The presence of a G/C heterozygous genotype at position-418 in the promoter of the TIMP-2 gene has been proposed as a genetic predisposing factor for MMD, but this association is debated. Park et al. 55) support the data that the G/C heterozygous genotype in the TIMP-2-418 G>C (rs8179090) promoter, MMP-2-1575GA/-1306CC, and the dominant type (GG vs. GA+AA) of MMP 9 Q279R (rs17576) could be predisposing genetic factors for MMD development. Vascular endothelial growth factor (VEGF) is involved in vasculogenesis in different intracranial lesions 61), is an endothelial cell mitogen that induces transient vascular leakage, and is a potent angiogenic factor 69). VEGF also promotes angiogenesis in cerebral ischemia 25,61) and causes pathologic vessel formation 9). Takekawa et al. 68) reported increased VEGF expression in autopsy specimens from adults with MMD and Sakamoto et al. 61) reported that the total meningeal cellularity and VEGF expression in the dura of patients with MMD was significantly higher than in the dura of controls. In ischemic disease, cerebral angiogenesis is caused by the release of VEGF 9,26). VEGF affects vasculogenesis, endothelial cell proliferation and migration, vascular permeability, and stromal degradation through the activation of proteolytic enzymes that are involved in angiogenesis 21,50). VEGF binds its receptor tyrosine kinases, VEGF receptor-1 and VEGF receptor-2 [also known as kinase insert domain containing receptor, or kinase insert domain contaning receptor (KDR)] but KDR is the key receptor mediating angiogenesis 71) and is essential for endothelial cell survival and integrity 13). Park et al. 53) found the genotypes including the VEGF-634C allele had better collateral vessel formation after surgery. They suggest that VEGF or KDR polymorphisms influence MMD as well as the formation of synangiosis-induced collateral vessel after bypass surgery.
Endothelial-based molecules and genetic studies (nitric oxide, eNOS)
Endothelial nitric oxide synthase (eNOS)-derived nitric oxide (NO) is one of the principal molecules in vasoregulation 56). Endothelial NO is responsible for endothelium-dependent vasorelaxation, inhibition of leukocyte and platelet adhesion, attenuation of inflammatory mediators, and has a key role in vasodilatory regulation of vascular smooth cells 49,60). Since NO is produced by eNOS, an understanding of eNOS (also known as NOS3) polymorphisms may help to explain variation in the clinical aspects of MMD. Park et al. 56) show that the haplotype a-4b-G was frequently found in patients with adult-onset MMD. These genetic differences can affect age-specific clinical characteristics such as cerebral ischemia and hemorrhage 56).
Smooth muscle cell-based genetic studies [Alpha actin 2 (ACTA2)]
The major function of vascular smooth muscle cells (SMCs) is to contract in response to the stretch resulting from pulsatile blood flow, a process that is dependent on the cyclic interaction between thin filaments, composed of the SMC-specific isoform of α-actin (SM α-actin, encoded by ACTA2), and thick filaments, composed of SMC-specific β-myosin 14). ACTA2 mutations associated with MMD provide further evidence that early-onset strokes may occur via a similar pathway of excessive SMC proliferation leading to arterial occlusion 14,57).
Atherosclerosis
Thromboembolic mechanisms, as well as hemodynamic instability in patients with MMD, play roles in cerebral infarction 54). An autopsy study of patients with MMD showed a frequent histopathology of thrombus formation in the diseased arteries 77). Prothrombotic disorders are associated with MMD in up to 40% of pediatric patients 7), and several studies have investigated the thromboembolic etiology in patients with MMD 2,5465). An association between ischemic stroke and a specific polymorphism in methylene tetrahydrofolate reductase (MTHFR; 677C>T) in children has been reported 29,44), and homozygous 677C>T in the MTHFR gene has been reported in patients with MMD 2,65). Park et al. 54) found the recessive type of MTHFR 677C>T and the C677T/A1298C compound genotype are significantly associated with adult MMD. They also found the frequency of the CT/AA sequence of MTHFR 677/1298 is significantly higher in MMD patients than in control subjects, especially in the hemorrhagic type of MMD 54). Thrombotic or thromboembolic as well as hemodynamic unbalance play roles in developing infarction in patients with MMD 10,2876).
Cytokines and growth factors
Several studies have found alterations in cytokines and growth factors in patients with MMD. The concentration of basic fibroblast growth factor (bFGF) in CSF has been shown to be elevated in patients with MMD compared to controls 43,78). Other studies have found increased immunoreactivity of bFGF in the dura mater 20), superficial temporal artery 18,20), and the circle of Willis 14) of MMD patients. Significantly elevated expression of cellular retinoic acid-binding protein (CRABP1) was found in the CSF of MMD patients 30). In vitro studies of vascular smooth muscle cells (VSMCs) from MMD patients revealed alterations in the cellular response to a platelet derived growth factor (PDGF) stimulus, most probably caused by a decreased amount of PDGF receptors 3,2275). Finally, higher concentrations of transforming growth factor beta 1 (TGFB1) were found in the blood serum and VSMCs of MMD patients 18,78).
Ring finger protein 213 (RNF 213)
Three individual studies of MMD patients have revealed high frequencies of the same single base substitution (nonsynonymous mutation), the c.14576G>A (p.R4859K) variant in RFP213 (a gene located in chromosome 17q) 27,4046). The c.14576G>A in RNF213 is present in ~2% of East Asian populations, a relatively higher rate compared with Caucasians 27,4046). The RNF213 gene was further reported to correlate with the early-onset and severe forms of MMD, which indicates its value as a good biomarker for predicting prognosis 46). The RNF213 gene encodes a protein with 5256 amino acids harboring a RING (Really Interesting New Gene) finger motif and an AAA (ATPase associated with a variety of cellular activities) domain, indicating the presence of both E3 ubiquitin ligase activity and an energy-dependent unfoldase. E3 ubiquitin ligase, which has several subtypes, is an enzyme that ubiquitinates specific target proteins, resulting in degradation by proteasomes 48). The RNF213 variant associated with MMD prevails, but it is also found in other vascular diseases such as cerebrovascular stenosis 48), but not in the Caucasian MMD population 41). In RNF213-deficient mice, an abnormal vascular network does not develop at the base of the brain 66). The RNF213 variant is an important SNP, but cannot be specific to MMD only. Genome-wide association study (GWAS) approaches are now being applied to MMD with the hope of uncovering the underlying pathogenic mechanisms 1). A GWAS was recently performed in Japanese MMD patients and found a strong association of MMD risk with chromosome 17q25-ter 27). These GWAS studies will need further investigation to solidly replicate the results using modern genetic studies based on familial or non-familial MMD.
LIMITATIONS
SNP studies have some limitations. First, most studies lack long-term follow up, which is necessary to assess clinical outcomes. The second limitation is a lack of well-defined patient and control groups. Third, genetic studies have been carried out based on a small number of case-control studies. Large population-based case-control or analyses centered on family-based designs are needed. However, SNPs studies have many advantages over other genetic studies, the benefits of which depend on how SNPs will be exploited in relevant study designs and what traits and diseases will be the focus of these studies 63). We have considered some of the unique aspects of SNPs and their relative advantages and disadvantages in human population-based analyses 63). Although progress in the search for genetic loci underlying MMD is encouraging, a relevant, specific single gene has not yet been identified. MMD appears to be a multifactorial, polygenic disorder that does not display a classic pattern of inheritance.
CONCLUSIONS
There are several studies of the association of SNPs and MMD, which focus on hemodynamic stress, the endothelium, smooth muscle, atherosclerosis, cytokines, growth factors, and RNF 213. Current SNP studies suggest a genetic contribution to MMD, but further reliable and replicable data are needed. A large cohort or family-based design will be necessary. I believe that modern MMD SNP studies depend on novel genetic, experimental, and database methods and will lead to a better understanding of MMD.
Acknowledgements
This work was supported by the National Research Foundation of Korea (2013R1A2A2A01067990).
References
1. Achrol AS, Guzman R, Lee M, Steinberg GK : Pathophysiology and genetic factors in moyamoya disease. Neurosurg Focus 2009, 26 : E4,  2. Andreone V, Ciarmiello A, Fusco C, Ambrosanio G, Florio C, Linfante I : Moyamoya disease in Italian monozygotic twins. Neurology 1999, 53 : 1332-1335,   3. Aoyagi M, Fukai N, Sakamoto H, Shinkai T, Matsushima Y, Yamamoto M, et al : Altered cellular responses to serum mitogens, including platelet-derived growth factor, in cultured smooth muscle cells derived from arteries of patients with moyamoya disease. J Cell Physiol 1991, 147 : 191-198,   4. Aoyagi M, Ogami K, Matsushima Y, Shikata M, Yamamoto M, Yamamoto K : Human leukocyte antigen in patients with moyamoya disease. Stroke 1995, 26 : 415-417,   5. Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al : Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 2001, 21 : 7724-7232,    6. Baba T, Houkin K, Kuroda S : Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry 2008, 79 : 900-904,   7. Bonduel M, Hepner M, Sciuccati G, Torres AF, Tenembaum S : Prothrombotic disorders in children with moyamoya syndrome. Stroke 2001, 32 : 1786-1792,   8. Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR : Moyamoya disease : a summary. Neurosurg Focus 2009, 26 : E11,  9. Cao Y, Hong A, Schulten H, Post MJ : Update on therapeutic neovascularization. Cardiovasc Res 2005, 65 : 639-648,   10. Cho HJ, Jung YH, Kim YD, Nam HS, Kim DS, Heo JH : The different infarct patterns between adulthood-onset and childhood-onset moyamoya disease. J Neurol Neurosurg Psychiatry 2011, 82 : 38-40,   11. Fujimura M, Watanabe M, Narisawa A, Shimizu H, Tominaga T : Increased expression of serum Matrix Metalloproteinase-9 in patients with moyamoya disease. Surg Neurol 2009, 72 : 476-480, discussion 480   12. Fukui M : Current state of study on moyamoya disease in Japan. Surg Neurol 1997, 47 : 138-143,   13. Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al : Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998, 273 : 30336-30343,   14. Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, et al : Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet 2009, 84 : 617-627,    15. Han DH, Kwon OK, Byun BJ, Choi BY, Choi CW, Choi JU, et al : A co-operative study : clinical characteristics of 334 Korean patients with moyamoya disease treated at neurosurgical institutes (1976-1994). The Korean Society for Cerebrovascular Disease. Acta Neurochir (Wien) 2000, 142 : 1263-1273, discussion 1273-1274   16. Han DH, Nam DH, Oh CW : Moyamoya disease in adults : characteristics of clinical presentation and outcome after encephalo-duro-arterio-synangiosis. Clin Neurol Neurosurg 1997, 99( Suppl 2):S151-S155,   17. Han H, Pyo CW, Yoo DS, Huh PW, Cho KS, Kim DS : Associations of Moyamoya patients with HLA class I and class II alleles in the Korean population. J Korean Med Sci 2003, 18 : 876-880,    18. Hojo M, Hoshimaru M, Miyamoto S, Taki W, Nagata I, Asahi M, et al : Role of transforming growth factor-beta1 in the pathogenesis of moyamoya disease. J Neurosurg 1998, 89 : 623-629,   19. Hong SH, Wang KC, Kim SK, Cho BK, Park MH : Association of HLA-DR and -DQ genes with familial Moyamoya disease in Koreans. J Korean Neurosurg Soc 2009, 46 : 558-563,    20. Hoshimaru M, Takahashi JA, Kikuchi H, Nagata I, Hatanaka M : Possible roles of basic fibroblast growth factor in the pathogenesis of moyamoya disease : an immunohistochemical study. J Neurosurg 1991, 75 : 267-270,   21. Ikeda E, Achen MG, Breier G, Risau W : Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem 1995, 270 : 19761-19766,   22. Ikeda H, Sasaki T, Yoshimoto T, Fukui M, Arinami T : Mapping of a familial moyamoya disease gene to chromosome 3p24.2-p26. Am J Hum Genet 1999, 64 : 533-537,    23. Inoue TK, Ikezaki K, Sasazuki T, Matsushima T, Fukui M : Linkage analysis of moyamoya disease on chromosome 6. J Child Neurol 2000, 15 : 179-182,   24. Inoue TK, Ikezaki K, Sasazuki T, Ono T, Kamikawaji N, Matsushima T, et al : DNA typing of HLA in the patients with moyamoya disease. Jpn J Hum Genet 1997, 42 : 507-515,   25. Issa R, Krupinski J, Bujny T, Kumar S, Kaluza J, Kumar P : Vascular endothelial growth factor and its receptor, KDR, in human brain tissue after ischemic stroke. Lab Invest 1999, 79 : 417-425,  26. Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA : Induction of vascular endothelial growth factor and hypoxia-inducible factor-1alpha by global ischemia in rat brain. Neuroscience 2000, 99 : 577-585,   27. Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, et al : A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet 2011, 56 : 34-40,   28. Kastrup A, Schulz JB, Mader I, Dichgans J, Küker W : Diffusion-weighted MRI in patients with symptomatic internal carotid artery disease. J Neurol 2002, 249 : 1168-1174,   29. Kim SH, Hwang H, Chae JH, Kim KJ, Hwang YS, Lim BC : Ischemic stroke in a 7-month-old infant with antiphospholipid antibody and homozygous C677T methylenetetrahydrofolate reductase (MTHFR) polymorphism. J Child Neurol 2010, 25 : 1047-1050,   30. Kim SK, Yoo JI, Cho BK, Hong SJ, Kim YK, Moon JA, et al : Elevation of CRABP-I in the cerebrospinal fluid of patients with Moyamoya disease. Stroke 2003, 34 : 2835-2841,   31. Kitahara T, Okumura K, Semba A, Yamaura A, Makino H : Genetic and immunologic analysis on moya-moya. J Neurol Neurosurg Psychiatry 1982, 45 : 1048-1052,    32. Kraemer M, Horn PA, Roder C, Khan N, Diehl RR, Berlit P, et al : Analysis of human leucocyte antigen genes in Caucasian patients with idiopathic moyamoya angiopathy. Acta Neurochir (Wien) 2012, 154 : 445-454,   33. Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y : Research Committee on Moyamoya Disease in JapanRadiological findings, clinical course, and outcome in asymptomatic moyamoya disease : results of multicenter survey in Japan. Stroke 2007, 38 : 1430-1435,   34. Kuroda S, Houkin K : Moyamoya disease : current concepts and future perspectives. Lancet Neurol 2008, 7 : 1056-1066,   35. Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL : Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 2004, 35 : 1715-1719,   36. Lee SR, Lo EH : Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab 2004, 24 : 720-727,   37. Li H, Zhang ZS, Liu W, Yang WZ, Dong ZN, Ma MJ, et al : Association of a functional polymorphism in the MMP-3 gene with Moyamoya Disease in the Chinese Han population. Cerebrovasc Dis 2010, 30 : 618-625,   38. Liu C, Roder C, Schulte C, Kasuya H, Akagawa H, Nishizawa T, et al : Analysis of TGFB1 in European and Japanese Moyamoya disease patients. Eur J Med Genet 2012, 55 : 531-534,   39. Liu W, Hashikata H, Inoue K, Matsuura N, Mineharu Y, Kobayashi H, et al : A rare Asian founder polymorphism of Raptor may explain the high prevalence of moyamoya disease among East Asians and its low prevalence among Caucasians. Environ Health Prev Med 2010, 15 : 94-104,   40. Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, et al : Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One 2011, 6 : e22542,    41. Liu W, Senevirathna ST, Hitomi T, Kobayashi H, Roder C, Herzig R, et al : Genomewide association study identifies no major founder variant in Caucasian moyamoya disease. J Genet 2013, 92 : 605-609,   42. Lo EH, Dalkara T, Moskowitz MA : Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003, 4 : 399-415,   43. Malek AM, Connors S, Robertson RL, Folkman J, Scott RM : Elevation of cerebrospinal fluid levels of basic fibroblast growth factor in moyamoya and central nervous system disorders. Pediatr Neurosurg 1997, 27 : 182-189,   44. McKasson MJ, Golomb MR : Two children with both arm ischemia and arterial ischemic stroke during the perinatal period. J Child Neurol 2011, 26 : 1548-1554,   45. Mineharu Y, Liu W, Inoue K, Matsuura N, Inoue S, Takenaka K, et al : Autosomal dominant moyamoya disease maps to chromosome 17q25.3. Neurology 2008, 70( 24 Pt 2):2357-2363,   46. Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, et al : Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology 2012, 78 : 803-810,   47. Miyatake S, Touho H, Miyake N, Ohba C, Doi H, Saitsu H, et al : Sibling cases of moyamoya disease having homozygous and heterozygous c.14576G>A variant in RNF213 showed varying clinical course and severity. J Hum Genet 2012, 57 : 804-806,   48. Miyawaki S, Imai H, Takayanagi S, Mukasa A, Nakatomi H, Saito N : Identification of a genetic variant common to moyamoya disease and intracranial major artery stenosis/occlusion. Stroke 2012, 43 : 3371-3374,   49. Moncada S, Higgs A : The L-arginine-nitric oxide pathway. N Engl J Med 1993, 329 : 2002-2012,   50. Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP : Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 1995, 375 : 577-581,   51. Nanba R, Kuroda S, Tada M, Ishikawa T, Houkin K, Iwasaki Y : Clinical features of familial moyamoya disease. Childs Nerv Syst 2006, 22 : 258-262,   52. Nanba R, Tada M, Kuroda S, Houkin K, Iwasaki Y : Sequence analysis and bioinformatics analysis of chromosome 17q25 in familial moyamoya disease. Childs Nerv Syst 2005, 21 : 62-68,   53. Park YS, Jeon YJ, Kim HS, Chae KY, Oh SH, Han IB, et al : The role of VEGF and KDR polymorphisms in moyamoya disease and collateral revascularization. PLoS One 2012, 7 : e47158,    54. Park YS, Jeon YJ, Kim HS, Han IB, Choi JU, Kim DS, et al : The roles of methylenetetrahydrofolate reductase 677C>T and 1298A>C polymorphisms in moyamoya disease patients. Childs Nerv Syst 2014, 30 : 1687-1695,   55. Park YS, Jeon YJ, Kim HS, Han IB, Oh SH, Kim DS, et al : The GC + CC genotype at position -418 in TIMP-2 promoter and the -1575GA/-1306CC genotype in MMP-2 is genetic predisposing factors for prevalence of moyamoya disease. BMC Neurol 2014, 14 : 180,     56. Park YS, Min KT, Kim TG, Lee YH, Cheong HJ, Yeom IS, et al : Age-specific eNOS polymorphisms in moyamoya disease. Childs Nerv Syst 2011, 27 : 1919-1926,   57. Roder C, Peters V, Kasuya H, Nishizawa T, Wakita S, Berg D, et al : Analysis of ACTA2 in European Moyamoya disease patients. Eur J Paediatr Neurol 2011, 15 : 117-122,   58. Roder C, Peters V, Kasuya H, Nishizawa T, Takehara Y, Berg D, et al : Common genetic polymorphisms in moyamoya and atherosclerotic disease in Europeans. Childs Nerv Syst 2011, 27 : 245-252,   59. Roder C, Peters V, Kasuya H, Nishizawa T, Takehara Y, Berg D, et al : Polymorphisms in TGFB1 and PDGFRB are associated with Moyamoya disease in European patients. Acta Neurochir (Wien) 2010, 152 : 2153-2160,   60. Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW : Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg 2004, 26 : 1002-1014,   61. Sakamoto S, Kiura Y, Yamasaki F, Shibukawa M, Ohba S, Shrestha P, et al : Expression of vascular endothelial growth factor in dura mater of patients with moyamoya disease. Neurosurg Rev 2008, 31 : 77-81, discussion 81   62. Sakurai K, Horiuchi Y, Ikeda H, Ikezaki K, Yoshimoto T, Fukui M, et al : A novel susceptibility locus for moyamoya disease on chromosome 8q23. J Hum Genet 2004, 49 : 278-281,   63. Schork NJ, Fallin D, Lanchbury JS : Single nucleotide polymorphisms and the future of genetic epidemiology. Clin Genet 2000, 58 : 250-264,   64. Scott RM : Arteriovenous malformation and moyamoya disease. Childs Nerv Syst 1997, 13 : 357,   65. Skardoutsou A, Voudris KA, Mastroyianni S, Vagiakou E, Magoufis G, Koukoutsakis P : Moya moya syndrome in a child with pyruvate kinase deficiency and combined prothrombotic factors. J Child Neurol 2007, 22 : 474-478,   66. Sonobe S, Fujimura M, Niizuma K, Nishijima Y, Ito A, Shimizu H, et al : Temporal profile of the vascular anatomy evaluated by 9.4-T magnetic resonance angiography and histopathological analysis in mice lacking RNF213 : a susceptibility gene for moyamoya disease. Brain Res 2014, 1552 : 64-71,   67. Starke RM, Komotar RJ, Connolly ES : Optimal surgical treatment for moyamoya disease in adults : direct versus indirect bypass. Neurosurg Focus 2009, 26 : E8,  68. Takekawa Y, Umezawa T, Ueno Y, Sawada T, Kobayashi M : Pathological and immunohistochemical findings of an autopsy case of adult moyamoya disease. Neuropathology 2004, 24 : 236-242,   69. Thomas KA : Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem 1996, 271 : 603-606,   70. Wakai K, Tamakoshi A, Ikezaki K, Fukui M, Kawamura T, Aoki R, et al : Epidemiological features of moyamoya disease in Japan : findings from a nationwide survey. Clin Neurol Neurosurg 1997, 99( Suppl 2):S1-S5,  71. Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH : Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1994, 269 : 26988-26995,   72. Wang X, Zhang Z, Liu W, Xiong Y, Sun W, Huang X, et al : Impacts and interactions of PDGFRB, MMP-3, TIMP-2, and RNF213 polymorphisms on the risk of Moyamoya disease in Han Chinese human subjects. Gene 2013, 526 : 437-442,   73. Weinberg DG, Arnaout OM, Rahme RJ, Aoun SG, Batjer HH, Bendok BR : Moyamoya disease : a review of histopathology, biochemistry, and genetics. Neurosurg Focus 2011, 30 : E20,   74. Wu Z, Jiang H, Zhang L, Xu X, Zhang X, Kang Z, et al : Molecular analysis of RNF213 gene for moyamoya disease in the Chinese Han population. PLoS One 2012, 7 : e48179,    75. Yamamoto M, Aoyagi M, Fukai N, Matsushima Y, Yamamoto K : Differences in cellular responses to mitogens in arterial smooth muscle cells derived from patients with moyamoya disease. Stroke 1998, 29 : 1188-1193,   76. Yamashita M, Oka K, Tanaka K : Histopathology of the brain vascular network in moyamoya disease. Stroke 1983, 14 : 50-58,   77. Yamauchi T, Tada M, Houkin K, Tanaka T, Nakamura Y, Kuroda S, et al : Linkage of familial moyamoya disease (spontaneous occlusion of the circle of Willis) to chromosome 17q25. Stroke 2000, 31 : 930-935,   78. Yoshimoto T, Houkin K, Takahashi A, Abe H : Angiogenic factors in moyamoya disease. Stroke 1996, 27 : 2160-2165,  
Table 1
Reported genetic studies on Moyamoya disease
|
|
















