Management of Pediatric Intracranial Arteriovenous Malformations
Article information
Abstract
Pediatric intracranial arteriovenous malformations (AVMs) are challenging lesions managed by pediatric neurosurgeons. The high risk of hemorrhage and neurologic injury is compounded by the unique anatomy of each malformation that requires individualizing treatment options. This article reviews the current status of pediatric AVM epidemiology, pathophysiology and clinical care, with a specific focus on the rationale and methodology of surgical resection.
INTRODUCTION
Vascular malformations of the central nervous system (CNS) can be classified according to rheologic characteristics (fast-flow and slow-flow) and by channel composition, i.e., arteriovenous malformation (AVM), capillary malformation (CM), and venous malformation (VM) and may occur either independently or in association with syndromic conditions. AVM is arguably the most important vascular anomaly in the nervous system in children; it is relatively common and usually requires treatment.
PEDIATRIC EPIDEMIOLOGY AND PATHOPHYSIOLOGY
AVM is the most common symptomatic intracranial vascular abnormality [12]. In a large autopsy series the overall frequency of detection for AVMs was 1.4% (46 among 3200 brain tumor cases) [59]. In another report, the annual incidence of symptomatic AVMs was 1.1 per 100000 and about one-in-five AVMs will present in children [16,36]. CNS AVMs are present in about 1/5000 children with no sex preference and represent about one sixth of AVMs in the general population [13,14,40,41].
AVMs of the CNS are largely sporadic, but familial AVMs – often with multiple lesions in a given patient – can be present in hereditary hemorrhagic telangiectasia (HHT) or mutations in RASA-1 [9,36]. HHT often has germline mutations in ACVRL1 or ENG, while spontaneous AVMs are associated with mutations in KRAS, BRAF and other molecules in the ephrin (EPHB4) pathway [26,42]. These mutations often share a common end pathway activating RAS, leading to poor vessel tube formation and dysregulated migration – helping to shed light on the pathophysiology of AVM.
The primary high-flow lesion implicated in a spontaneous intracranial hemorrhage (ICH) in pediatric patients is an AVM [3,4,7,11,39,51]. Each bleed has a 12–25% probability of fatality, and rate of bleeding is substantially higher after hemorrhage, which have been estimated at 6% in the immediate period after bleeding for the first 6 months, versus 1–6% annually if there was no bleeding [5,17,24,28,51]. These sobering data validate aggressive approaches to treatment in many cases. Children may also experience seizure, headache and loss of function secondary to pressure from the vessels or hemodynamic steal [27,32,35,38]. It is presumed that the majority of AVMs are asymptomatic—although not without hemorrhagic risk, making management of incidentally found AVMs controversial.
DIAGNOSIS
As with all CNS diseases, a thorough review of systems, bedside examination and dedicated imaging are first steps in the diagnostic effort [58]. As most children present with symptoms, the nature of the findings should guide the speed and approach of the workup. In an emergency, particularly when a bleed is suspected, the use of computed tomography (CT) and CT angiography (CTA) is an expedient and highly informative first test [75].
Diagnosis is typically initially made with CTA or – if not emergent – magnetic resonance imaging and angiography (MRI/MRA), and subsequent digital subtraction angiography (DSA) should be considered in most cases, as it offers the greatest resolution of nidal anatomy, with CTA having 91% accuracy compared to DSA in the setting of spontaneous ICH [64,81].
DSA is low risk and high yield. Analysis of pediatric patients revealed only a 0.4% post-procedural complication rate [50,60]. Up to 15% of cerebral AVMs receive some blood supply from ipsilateral or contralateral meningeal arteries, typically not visible on MRA [57]. Importantly, DSA can offer important predictive data on the risk of hemorrhage, including outflow stenosis, smaller size and deep venous drainage [21,66]. Because blood and swelling from rupture may hide parts of the AVM by compression or spasm, angiography may be delayed or repeated weeks later to ensure nothing is missed [19].
As noted in the prior section, a small fraction of patients may have germline mutations that can cause multiple lesions and/or other related findings. If a child has small telangiectasias, CMs, asymmetric limbs or more than one AVM seen on imaging, a genetic workup for RASA-1 or HHT related mutations should be undertaken [45,77]. Genetic counselors can be helpful in this effort.
CLASSIFICATION METHODS FOR INTRACRANIAL AVMS
Once an AVM has been identified, it is helpful to understand the potential risks of various treatment options. Consequently, a number of grading and classification schema have been developed.
Microsurgery
Understanding the potential risk of surgery is important for both patient and treating physician. The Spetzler-Martin system incorporates bigger size (<3 cm, 3–6 cm, and >6 cm), eloquence of adjacent brain and presence of deep venous drainage as factors that adversely impact outcome from surgery [31,71]. The total possible score is 5 points and each point adds approximately 10% greater risk of deficits. Follow-up papers in 2010 and 2011 added a tiering system (graded A–C, from lower to higher risk) and additional points incorporating unruptured status, age (older being greater risk) and whether the nidus was compact (lower risk) or diffuse (higher risk) [47,48,72].
Radiation
The Radiosurgery based AVM grading scale (RBAS) is currently well-adopted [6]. The RBAS calculates a score with this formula : 0.1 × volume (in cm3) + 0.02 × age (in years) + 0.5 × location (1 for basal ganglia, brain stem, or thalamus) [61,63,78]. This score is then associated with outcome that both includes extent of obliteration at a median follow-up of 39 months, and occurrence of complication. When the score is broken down into four groups (1 or less, 1–1.5, 1.5–2, and more than 2), it is correlated with obliteration rates of 62%, between 51–53%, and as low as 32%, all without complications. The Virginia score considers the AVM size (0–2 points, with higher assigned to larger lesions), eloquence (1 point), and history of hemorrhage (1 point) [74]. Each point has the same value and the cumulative score correlates with a proportion of favorable outcome (obliteration of AVM without post-treatment hemorrhage and without treatment-associated symptoms) from 83% to 39%. An additional system is the Pollock-Flickinger score, used in some centers to predict patient outcomes after radiosurgery [62].
Intervention
Several scoring systems have been proposed since 2010 for predicting response to embolization, but vary greatly [18,22,30,37,52,65]. The Puerto Rico score incorporates arterial feeders (0–6, with more feeders assigned a higher score), whether the adjacent brain has eloquence, and if an arteriovenous fistula is present [22]. Another system from Buffalo is very similar, adding the factor of looking at the size of the pedicles as measured by diameter [18]. Lastly the AVM embocure score adds in consideration of two factors – how many draining veins are present and the size of the nidus (bigger being greater risk) [52]. It is worth noting that these reports came prior to the widespread use of transvenous approaches, and future efforts may need to account for these evolutions.
INDICATIONS FOR TREATMENT
Most centers endorse an aggressive approach toward the treatment of AVMs in the pediatric population, due to devastating impact of bleeding and cognizance that the long lifespan of a child confers a greater cumulative risk over time. Fundamental to any treatment is the acknowledgement that the primary endpoint of an intervention is total shutdown or removal of the AVM, as anything less than total cure means that the lesion can recanalize in the future and – with this return of flow – still kill the patient by rupture [58]. This treatment can be open resection with surgery, radiation to induce fibrosis/thrombosis or embolization to occlude nidal vasculature. It is important to note that in some complex cases, careful assessment by the medical team may determine that the “cure may be worse than the disease,” and observation may be recommended to offer better odds of a good quality of life. Given the individualized nature of AVMs – especially in the pediatric population with growing and developing patients – multimodality therapy may be especially important and referral to high-volume, experienced centers is encouraged [33,34,49,76].
In children, the first line therapy supported by the preponderance of evidence is surgical resection (with or without embolization) for most simple AVMs (Spetzler-Martin, 1–3), and this included unruptured, asymptomatic lesions in many cases, due to the remarkably high rates of cure (>95%) and plasticity of the pediatric nervous system in recovering from potential deficits [28,29,54]. Data from the ARUBA trial (A Randomised trial of Unruptured Brain Arteriovenous malformations - which assessed asymptomatic AVMs) when the pediatric cohort is isolated for analysis, demonstrates a significant benefit for surgical resection, with a childhood natural history worse than adults – supporting surgery as a first line therapy [28].
Stereotactic radiosurgery is well-established as a successful way of treating AVMs, and it is evident that there are a population of lesions that sit in a “gray zone” that can likely be treated with similar outcomes by either radiation or surgery. While not the focus of this review, radiosurgery can be an effective treatment for intracranial AVMs, with concerns centering largely on the concern for re-bleeding in hemorrhagic AVMs (as radiosurgery has a latent period of delay between treatment and cure) and the generally lower cure rates versus surgery. One of the benefits of radiosurgery is the ability to treat diffuse, large AVMs, with some literature suggesting modest success for these otherwise challenging lesions, including combined therapy [1,15,70]. In any radiosurgery case, the effect of radiation on adjacent brain – “radiation effect” – consisting of potential edema and tissue injury, should be considered and monitored.
Pediatric data suggests that embolization alone may increase the rate of post-treatment hemorrhage relative to the natural history, leading to the recommendation to avoid embolization as a stand-alone therapy for pediatric AVM [8,28,54]. However, in combination with other treatments, embolization is a critical component contributing to the successful multimodal management of brain AVMs that is utilized by interdisciplinary teams [29,50,67,79]. Overall, most pediatric AVMs should be considered for treatment, including incidentally found, asymptomatic lesions, including with data from the recent American Heart/Stroke Association guidelines [23].
Areas of evolution
One of the areas of controversy is the management of cerebral proliferative angiopathy (CPA). This entity differs from typical brain AVMs by its diffuse, large, slower-flow angioarchitecture and – clinically – appears to have a greater likelihood of causing ischemic injury [25,46]. Unlike more compact nidal AVMs (as reviewed in this manuscript), these CPA lesions are often considered to be unresectable, due to their large size and involvement with normal parenchyma. Given their potential to damage healthy brain due to steal with associated ischemic injury, a number of centers have started to advocate the use of revascularization surgery, such as pial synangiosis [20]. While seemingly counterintuitive to add blood supply to a vascular malformation, the hypothesis is that this operation can improve perfusion to eloquent brain adjacent to the lesion. This approach has had some encouraging initial success and remains under investigation.
SURGICAL MANAGEMENT OF PEDIATRIC AVMS
The decision on whether to treat an AVM and how to treat a given lesion is best done at high-volume centers, ideally where multimodality care can be employed if indicated [33,34,49,76]. This team approach, coupled with high-volume experience, has been reported to result in better outcomes [67]. As reviewed previously, surgery is generally recommended for most low- and moderate-risk AVMs found in children.
Decision on timing of surgery
It is obvious that the presentation of the AVM dictates the timing of intervention in the majority of cases. For asymptomatic lesions, because the risk of a bleed is relatively minimal for most lesions (approximately 1–3% per year for an unruptured AVM), many centers are comfortable with allowing weeks or even months to proceed through a workup. If there are worrisome radiographic features – rapid change in the size of the lesion, evidence of severe outflow stenosis or concerning aneurysms found with the AVM – then more urgent treatment may be warranted.
For those lesions that have bled, immediate surgery may be needed to relieve mass effect from a clot and/or manage hydrocephalus. In some emergencies, DSA and MRI may not be safe due to the time required to perform the study and CTA can be a rapid and informative imaging option [68]. If the AVM anatomy is unclear or the patient is unstable, a reasonable operative goal may center on controlling intracranial pressure (ICP) and deferring definitive resection of the AVM until a later time. Care should be taken to remove clot only, leaving the vascular malformation undisturbed. A helpful technique may include a large bone flap removal, followed by a very small dural slit to decompress clot in a controlled fashion using ultrasound guidance, followed by subsequent wide dural opening and exploration (Fig. 1). This method can reduce the risk of re-rupture from sudden loss of vessel tamponade potentially precipitated by immediate wide dural opening.
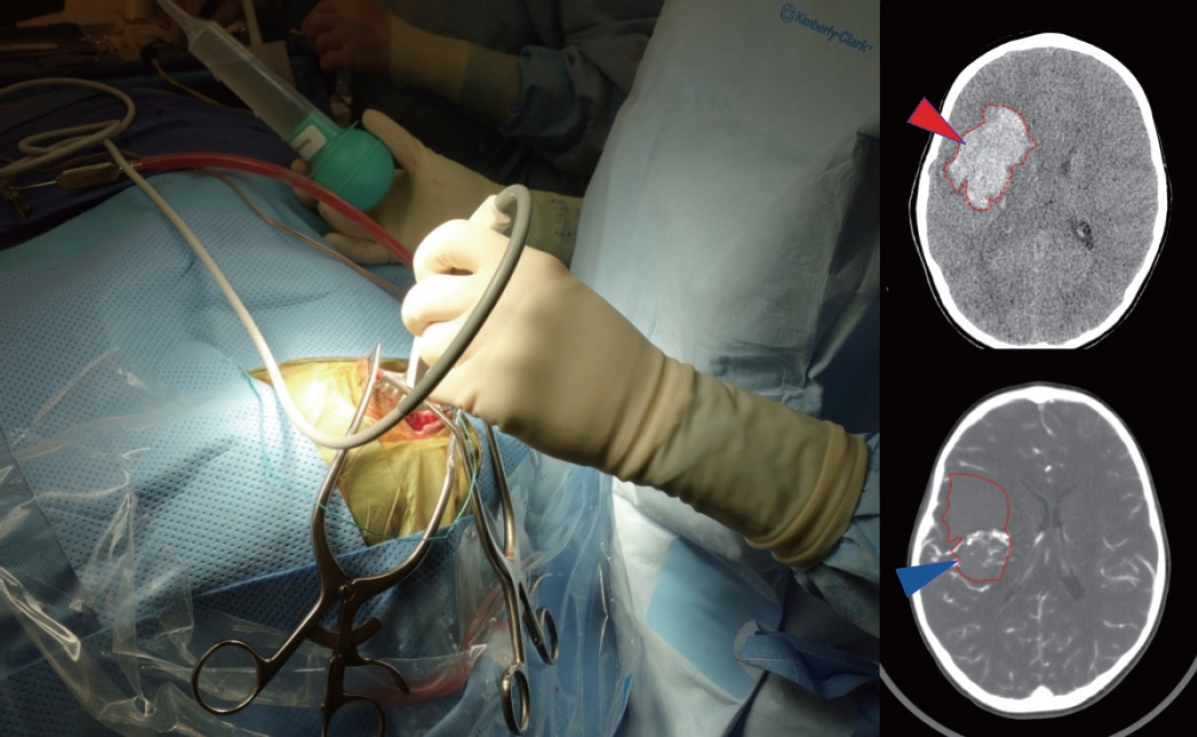
Use of an intraoperative ultrasound to help localize clot (red arrowhead on CT) and AVM nidus (blue arrowhead on CTA) in order to guide controlled decompression of the clot through a small dural opening in advance of a wider exposure. Using the Doppler flow settings can be particularly helpful to visualize AVM, especially when paired with CT/CTA studies. CT : computed tomography, AVM : arteriovenous malformation, CTA : computed tomography angiography.
Following decompression, definitive surgical treatment to resect the AVM may be scheduled in a more elective fashion, coincident with the imaging studies when the anatomy of the AVM can be delineated by formal angiography with decreased hematoma mass effect. Rebleeding rates are about 6% for the first 6 months and 3% per year afterwards, making timing of treatment in these cases more pressing – although many centers will allow several weeks for swelling to recede before operating if the child is otherwise well [27,80]. The judgment of the team and the clinical status of the child are critical to the individual decision-making around planning.
Perioperative and anesthetic considerations
There is little data around the specific risk of AVM rupture during the onset of anesthesia, but major swings in arterial blood pressure should be avoided as much as possible [58,82]. Of particular importance is the control of blood pressure during placement of cranial pin fixation to minimize risk of AVM rupture. Once induction is over and the case has started, anesthesia should communicate with the surgical team about how to minimize physiological perturbations that may affect bleeding or swelling throughout the procedure. Anesthesia should be ready for blood loss, with good intravenous access, blood available to transfuse and access to osmotic diuretics if needed.
With larger high-flow AVMs, the risk of hyperperfusion is greater, so steps to mitigate this problem – such as preoperative embolization to effect a stepwise re-routing of blood flow – can be helpful [69]. Postoperatively, careful blood pressure control and frequent neurological examinations are important.
Operative techniques
The profound variability in anatomy and clinical presentation for pediatric AVMs means that it is impossible to standardize operative approaches. Rather, the core principles described below should be applied. Overall, every AVM surgery should share general strategies but individualize tactics to conform to the unique aspects of the case.
Equipment should include the drill, a microscope, stereotactic guidance, ultrasound, multiple suctions, bipolar electrocautery, and vascular clips. One of the most critical parts of the surgery is the opening, since rupture can occur with removal of the bone flap or injury to vessels with dural dissection. Consequently, heightened attention during this stage, including pre-selecting appropriate clips and having the microscope sterile, is key. A large opening can be helpful to fully appreciate the relevant anatomy and deliberate, careful dural reflection is important to minimize the risk of inadvertent bleeding from damage caused by tearing adhesions to the AVM – and this sometimes means cutting windows of dura and leaving scarred areas alone until better control of the AVM is achieved. It is common to encounter a tight brain upon opening the dura. Key maneuvers such as elevating the head of the bed, mild hyperventilation or use of osmotic diuretics may help resolve this problem. Drainage of CSF can be useful, but it is important not to tear vessels while doing so.
Once the AVM is exposed, the following strategies are helpful in most cases, including : 1) a primary surgical principle for AVM resection is the obliteration of feeding arteries before occlusion of draining veins, as premature closure of outflow can lead to unexpected AVM rupture with uncontrolled bleeding. This is a key advantage and goal of pre-operative embolization when applied; some philosophical approaches eschew embolization but require violating the second principle and performing deep approaches early on to control the common deep arterial feeder. 2) AVMs are usually conical and awareness of the three-dimensional shape of the lesion is crucial to avoid veering into the nidus (Fig. 2). 3) The cone or linear shape of many pediatric AVMs often correlates with the base of the malformation contacting the ependyma of a ventricle, underscoring the need to ensure that the ventricle is seen at surgery to fully resect the lesion (Fig. 3) [53]. 4) When under the microscope, it is easy to get too focused on the immediate area of work; it is important to periodically zoom out to see the whole field and check for swelling, bleeding or inappropriate retraction that might be otherwise missed. 5) Nidal vessels are dysplastic and often do not coagulate well. It is recommended to stay out of the body of the AVM and manage bleeding with clips or gelfoam/cottonoid when possible. And 6) an intra/perioperative angiogram (DSA) is recommended to ensure complete resection [23].
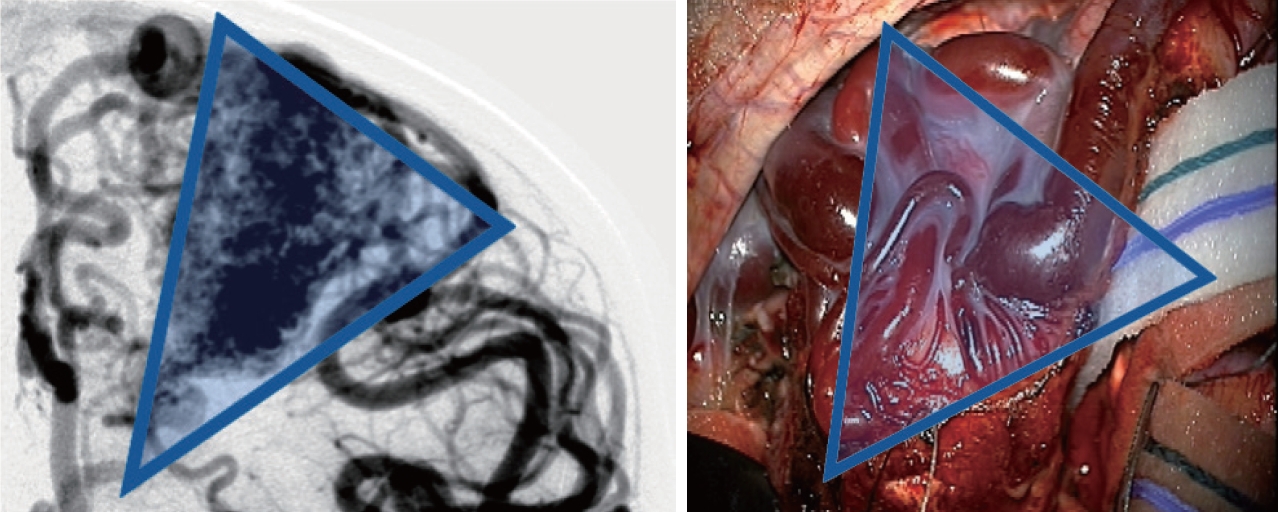
Angiographic correlation with intraoperative photograph highlighting conical shape of arteriovenous malformation, including both surface findings and surgical technique of working around the “cone” of the nidus, walling the margins off with cottonoids.
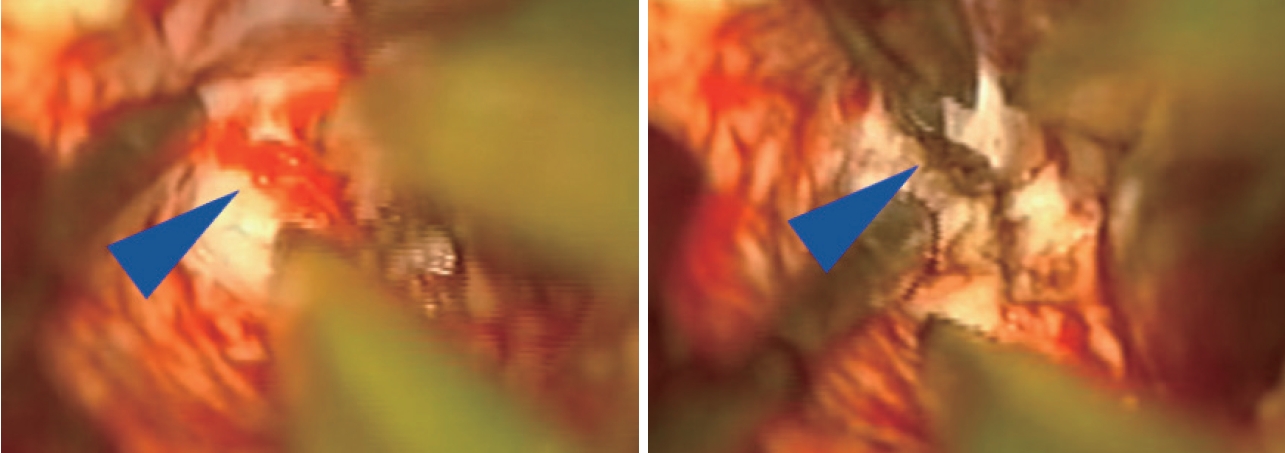
Intraoperative microscope images demonstrating small tuft of arterial vessels near ependyma of ventricle at base of arteriovenous malformation (left image, blue arrowhead) with subsequent cauterization and division of these feeders (right image). In addition, this image also highlights the utility of having an assistant working under the microscope to improve visualization and minimize prolonged retraction on adjacent brain.
Closure
After the concentration and effort expended in resecting an AVM, it is easy to be fatigued at the end of the case, which can lead to complications if not resisted. Blood in the ventricle may cause hydrocephalus and swelling may also result from unrecognized bleeding in the cavity, incomplete resection or hyperperfusion. Use of ultrasound (if available) can help identify these problems, as can a perioperative angiogram, which is recommended in the USA guidelines [23]. Closure can often be performed with a resorbable skin stitch, particularly helpful for children.
Complications
Intraoperative rupture with blood loss is the most feared risk, especially in the pediatric population that cannot tolerate volume loss like adults. There may be rapid decompensation in children, which mandates careful monitoring and replacement of blood products by the operative team.
Normal perfusion pressure breakthrough is a phenomenon that is thought to occur after resection of high flow AVMs. This can result in brain swelling, increased ICP, seizure, neurologic dysfunction or hemorrhage. The problem may be minimized by staged preoperative embolization and rigorous blood pressure control and sedation/anesthesia postoperatively.
Sometimes a normal artery is damaged with treatment, by off-target embolization or operative injury. Management focuses on improving supply by supporting blood flow to the region and adjacent collaterals, typically with blood pressure increases in the intensive care setting [83]. Obviously the benefits of this approach have to be weighed against the risk of rupture of an incompletely treated AVM, but it appears to be a well-tolerated strategy in most patients [58].
One of the most common complications after AVM surgery is a new neurological deficit. Many times these are anticipated preoperatively due to the location of the AVM and it can be important to communicate and set expectations with the patient and family before the case. In general, deficits for pediatric AVM resection are reported around 0–12% [3,33,34,43,55]. If present, evaluation with imaging, such as MRI, can be helpful, as well as assessing for functional problems like seizure or electrolyte imbalances, particularly sodium levels.
Outcomes and follow-up
It is difficult to generalize outcomes for AVMs, given the overwhelming heterogeneity of anatomy, clinical presentation, and plasticity of the developing brain. Overall, close to 95% of surgical cases demonstrate long-term cure, although recurrence can happen even years later and one of the most common permanent deficits is a visual field cut, reported in about one out of seven cases [28,29]. Comparing the efficacy of radiation for similar grade AVMs, roughly 75% long-term control is average [10,73].
A growing area of concern is the appreciation that children treated for AVMs can experience recurrence/regrowth, even years later, with as many as one in 10 cases affected and recurrence estimated at 1% after 5 years for all comers [2,29,56]. This emerging body of evidence has highlighted the value of ensuring complete removal at surgery, including the use of intra/peri-operative DSA (which has revealed up to 1/5th of cases may have unsuspected residual at time of surgery), as well as continued radiographic follow-up for the long term postoperatively, especially for AVMs with indistinct margins and those that presented with hemorrhage [23,29,44]. While a uniform protocol for following pediatric AVM patients remains in evolution, many centers consider annual imaging with MRI/MRA, complemented by DSA at greater intervals (often at 1 or 5 years postop) [8,29,56].
CONCLUSIONS
While pediatric AVMs are potentially devastating lesions, the rapid evolution of radiographic, endovascular, and surgical advances have substantially improved the prognosis of affected children. Critical to this ongoing progress is the need for collaboration across disciplines and leveraging talent and innovation by fostering the development of high-volume centers of excellence. As a surgeon, the most important decision is whether to operate or not. Maximize the odds for success by formulating a detailed surgical plan and fully engaging the entire operative team. In experienced hands, outcomes for pediatric AVMs can be excellent but require attentive long-term follow-up to ensure the durability of cure.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : ERS; Data curation : ERS, APS; Writing - original draft : APS; Writing - review & editing : ERS, APS
Data sharing
None
Preprint
None

