Feasibility of Gamma Knife Radiosurgery for Brain Arteriovenous Malformations According to Nidus Type
Article information
Abstract
Objective
Gamma Knife radiosurgery (GKRS) is an effective and noninvasive treatment for high-risk arteriovenous malformations (AVMs). Since differences in GKRS outcomes by nidus type are unknown, this study evaluated GKRS feasibility and safety in patients with brain AVMs.
Methods
This single-center retrospective study included patients with AVM who underwent GKRS between 2008 and 2021. Patients were divided into compact- and diffuse-type groups according to nidus characteristics. We excluded patients who performed GKRS and did not follow-up evaluation with magnetic resonance imaging or digital subtraction angiography within 36 months from the study. We used univariate and multivariate analyses to characterize associations of nidus type with obliteration rate and GKRS-related complications.
Results
We enrolled 154 patients (mean age, 32.14±17.17 years; mean post-GKRS follow-up, 52.10±33.67 months) of whom 131 (85.1%) had compact- and 23 (14.9%) diffuse-type nidus AVMs. Of all AVMs, 89 (57.8%) were unruptured, and 65 (42.2%) had ruptured. The mean Spetzler-Martin AVM grades were 2.03±0.95 and 3.39±1.23 for the compact- and diffuse-type groups, respectively (p<0.001). During the follow-up period, AVM-related hemorrhages occurred in four individuals (2.6%), three of whom had compact nidi. Substantial radiation-induced changes and cyst formation were observed in 21 (13.6%) and one patient (0.6%), respectively. The AVM complete obliteration rate was 46.1% across both groups. Post-GKRS complication and complete obliteration rates were not significantly different between nidus types. For diffuse-type nidus AVMs, larger AVM size and volume (p<0.001), lower radiation dose (p<0.001), eloquent area location (p=0.015), and higher Spetzler-Martin grade (p<0.001) were observed.
Conclusion
GKRS is a safe and feasible treatment for brain AVMs characterized by both diffuse- and compact-type nidi.
INTRODUCTION
Brain arteriovenous malformation (AVM) is a cerebrovascular defect characterized by an abnormal high-flow, low-resistance shunt through a nidus without capillary involvement. Although the exact prevalence is difficult to estimate, studies report that AVMs occur in 15–18 cases per 100000 people [22]. The high flow rate through the AVM vascular system introduces a greater risk of intracranial hemorrhage than do other cerebrovascular malformations such as cavernous malformations, capillary telangiectasias, and developmental venous anomalies. Furthermore, local neurological deficits, including headaches and seizures, may occur regardless of hemorrhage. AVM treatment includes microsurgical resection, endovascular embolization, radiosurgery, or a combination of these strategies. The goal of treatment is to obliterate the AVM nidus to minimize the risk of hemorrhage without causing neurological impairment.
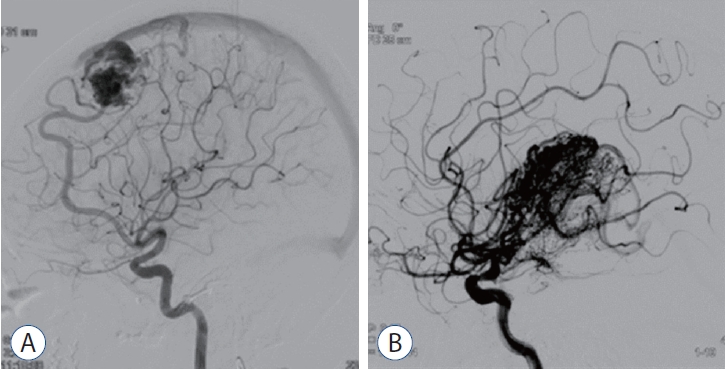
AVMs can be classified into two groups according to nidus features [1,14]. A compact-type nidus is a distinct, well-defined border. But some cases of AVM, its unclear boundaries interspersed throughout normal brain tissue so cannot be clearly recognizable. Previous study has proposed to name this kind of AVM for cerebral proliferative angiopathy, formerly known as the “diffuse-type nidi”. Diffuse-type nidi was defined as an atypical entity. It has histopathologically diffuse network of capillaries and venous ectasias in which normal brain lesion is present. And angioarchitecturely it has no dominant feeder or flow-related aneurysm. T1-weight magnetic resonance imaging (MRI) shows diffuse irregular enhancement and on perfusion studies an increase of blood volume and longer mean transit time due to diffuse capillaries in diffuse-type nidi. Consequently, diffuse-type nidi may impede complete AVM obliteration. Surgical resection risks injury to normal brain tissue, and diffuse-type nidus embolization may prove difficult [17,24]. Gamma Knife radiosurgery (GKRS) is an effective and noninvasive treatment option for surgically high-risk or complex AVMs [9]. However, complications of this approach include hemorrhage, radiation-induced changes (RIC), cyst formation, and radiation necrosis. This study evaluated the feasibility and safety of GKRS according to nidus type in patients with brain AVMs.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Ajou University Hospital (IRB-DB-2022-510). The requirement for patient consent was waived because of the retrospective nature of the study.
Patient population
We performed a retrospective study of demographic, clinical, neuroradiological imaging, and GKRS data from a prospectively maintained database of patients at a single-center hospital from October 2008 to December 2021. During the study period, a total of 193 patients underwent single-session GKRS, and 39 of them were excluded from the study. This exclusion was due to the fact that 30 patients were lost to outpatient follow-up or did not undergo imaging examinations after GKRS, and nine patients were observed after GKRS but had a follow-up duration of less than 36 months. Neuroradiologists and neurosurgeons had diagnosed AVM and determined whether treatment was warranted. The factors analyzed in our study included AVM nidus type, AVM location, size and volume, Gamma Knife radiation dose and target location, and previous treatment history. Moreover, we evaluated the patient’s Karnofsky performance scale (KPS) score before GKRS was performed. We divided AVMs into compact and diffuse types according to nidus characteristics. Using digital subtraction angiography (DSA) and MRI, criteria for determining the nidus type included the following : whether normal brain tissue was significantly included around the nidus, whether AVM boundaries could be easily distinguished, and whether a clear nidus feeder was observed and spread through adjacent tissue (Fig. 1). AVMs were further classified according to the Spetzler-Martin grading scale. The primary outcomes assessed were the complete obliteration rate and post-GKRS complications such as hemorrhage, RIC, and cyst formation.
Radiosurgery treatment strategy and protocol
Frame-based, thin-slice MRI was performed before and after treatment for all GKRS patients, and DSA and/or MRI were used to confirm nidus obliteration and the presence of any complications. The cerebrovascular surgeon and medical physicist discussed and determined the GKRS dosage and target AVM lesion with the goal of including as much of the AVM feeder as possible. A marginal dose of 15–16 Gy was regarded as the lowest dose required to obtain complete obliteration in >50% of the patients. We prescribed a marginal dose of 14–16 Gy for diffuse-type nidus and small AVMs. The marginal dose was decreased to 10–12 Gy for large AVMs and was used as the lowest dose to obtain any obliteration response. Gamma planning was performed under supervision, combining MR images (T1-weighted, T2-weighted, T1-enhanced) and DSA images obtained on the day of the procedure through fusion. In the case of diffuse nidus lesions, based on our experiences, we excluded draining veins and large vessels, and instead, we delineated the abnormal vasculature as extensively as possible, with a focus on the feeding artery, for segmentation within the radiation field. We configured it with a lower marginal dose, ensuring that the average dose (18.9±2.8 Gy) appropriately reached the feeder to achieve adequate planning. GKRS was conducted in a single session using the Leksell Gamma Knife® Model C or Icon™ (Elekta Solutions AB, Stockholm, Sweden) fixed by a Leksell Coordinate Frame. To assess AVM nidus features, we used stereotactic T1-weighted contrast-enhanced and T2-weighted MRI sequences, as well as DSA images.
Follow-up imaging
Follow-up imaging was performed at 6 months post-GKRS using MRI and magnetic resonance angiography, followed by annual MRI and DSA at year 3. MRI sequences included T1- and T2-weighted turbo spin echo, T2-weighted fluid-attenuated inversion recovery, diffusion-weighted imaging, and time-of-flight imaging. The disappearance of abnormal shading of the AVM nidus in DSA imaging was defined as complete obliteration. For cases in which DSA was not performed, complete obliteration was characterized by loss of flow void around the nidus in MRI. Based on the findings of previous studies, factors known to affect complete AVM obliteration and GKRS complications were analyzed.
Statistical analyses
Pearson’s chi-square test was used for categorical variates presented as mean and standard deviation, median and range, or percentage. The Kaplan-Meier method and log-rank test were used to determine the obliteration rate and associated factors. Univariate and multivariate analyses were conducted using Cox proportional hazard regression models. These data are presented as hazard ratios (HRs) and confidence intervals (CIs). A p-value <0.05 was deemed statistically significant. All analyses were performed using SPSS Statistics 25 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics
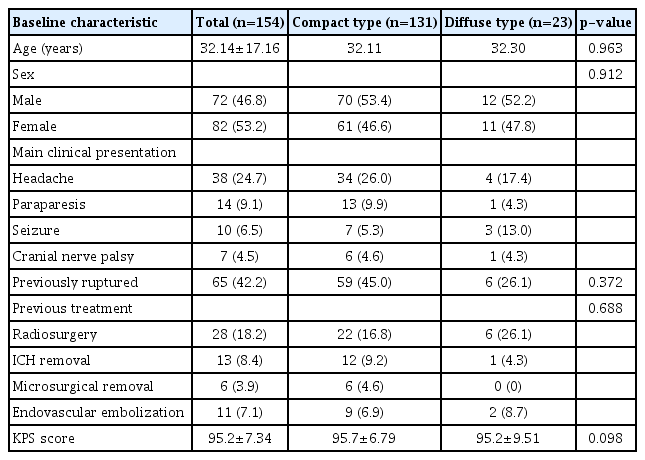
Between October 2008 and December 2021, 193 patients with cerebral AVMs received GKRS treatment at our hospital. We excluded 39 patients who performed GKRS and did not follow-up evaluation with MRI or DSA within 36 months from the study. Thus, 154 patients were included in the study (Table 1). Of these cases, 131 (85.1%) were classified as compact- and 23 (14.9%) as diffuse-type nidus AVM. Of all the patients, 72 were male (46.8%), and the mean age was 32.14±17.16 years. No significant differences regarding the age and sex of the two nidus groups were identified (p=0.963 and p=0.912, respectively). The primary clinical presentation was headache in both groups. Of the patients with compact-type nidus AVM, 34 (26.0%) presented with headache, which was comparable to four patients (17.4%) with diffuse-type nidus AVM. The numbers of patients in the compact- and diffusetype groups treated with GKRS after AVM rupture were 59 (45.0%) and six (26.1%), respectively. Among all the patients, 28 (18.2%) had undergone prior radiosurgery, 19 (12.3%) had received craniotomy with intracerebral hemorrhage removal surgery, and 11 patients (7.1%) had undergone embolization. No significant differences between the two groups with regard to previous treatment were identified (p=0.688). The mean KPS score for all patients was 95.2±7.34, indicating that the general condition of the patients did not significantly differ between the two groups before GKRS treatment (p=0.098).
AVM and GKRS characteristics
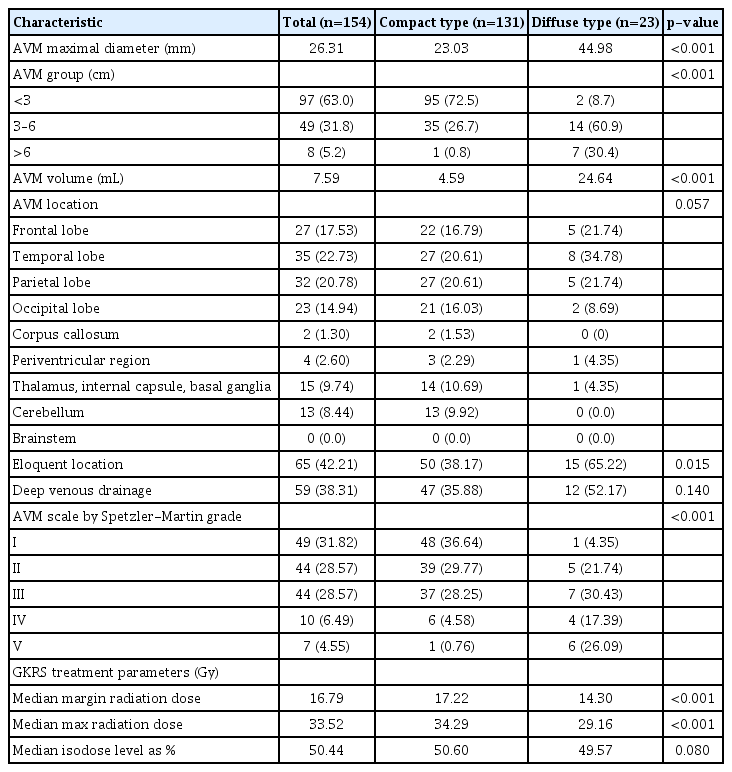
As shown in Table 2, the average maximum AVM diameter in patients who underwent GKRS was 26.31 mm. For diffuse nidi, the mean maximum AVM diameter was 44.98 mm, which was significantly different from that of compact nidi (23.03 mm, p<0.001). Similarly, the AVM size and volume of the diffuse-type group were larger than those of the compacttype group (AVM volume 24.64 vs. 4.59 mL, p<0.001). AVM locations from most to least common in the cohort were as follows : temporal lobe (22.73%), parietal lobe (20.78%), frontal lobe (17.53%), occipital lobe (14.94%), thalamus and basal ganglia (9.74%), cerebellum (8.44%), periventricular region (2.60%), and corpus callosum (1.30%; p=0.057). Compared with the compact-type nidus AVM, it can be interpreted that the diffuse-type nidus AVM location is mainly distributed in the eloquent region, including frontal, temporal, and parietal lobes. Diffuse-type nidi were distributed in eloquent locations significantly more often than were compact nidi (62.22% vs. 38.17%, p=0.015). Regarding deep venous drain pattern, 12 of 23 patients (52.17%) in the diffuse-type group showed a higher distribution with deep vein pattern than did 47 of 131 patients in the compact-type group (35.88%); however, this finding was not statistically significant (p=0.140). The median GKRS margin dose was 16.79 Gy for all patients, 17.22 Gy for the compact-type group, and 14.30 Gy for the diffuse-type group, exhibiting statistically significant differences between groups (p<0.001). This statistically differences can be interpreted as a result of setting the radiation dose to minimize complication while treating a large target lesion of the diffusetype group focused on feeding arteries.
Post-GKRS obliteration rate
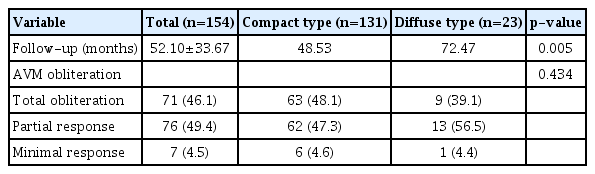
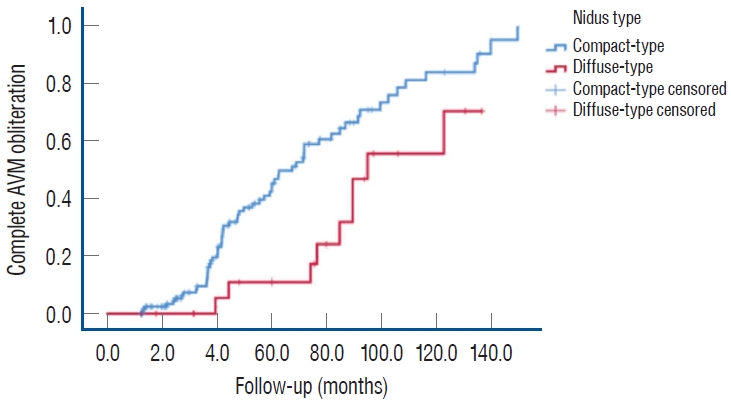
As shown in Table 3, the mean follow-up duration for the entire cohort was 52.10±33.67 months (range, 12.0–149.7). The follow-up durations for the diffuse- and compact-type groups were 72.47 and 48.53 months, respectively (p=0.005). The complete AVM obliteration rate was 46.1% in the entire patient group. Furthermore, this rate was 48.1% for compact and 39.1% for diffuse nidi (p=0.434, Fig. 2). Most patients showed a favorable response to GKRS, with only seven of 154 (4.5%) demonstrating minimal response to treatment.
Post-GKRS complications
Among all patients, RIC were the most common post-treatment complications, occurring in 21 of 154 individuals (13.6%). Hemorrhage occurred in four patients (2.6%), and there was only one case (0.6%) of cyst formation (Table 4). No statistically significant differences were identified between the two groups (p=0.133). Fig. 3 shows the complication that occurred after GKRS treatment. Among the four patients who experienced hemorrhage as a post-GKRS complication, one patient underwent additional partial glue embolization, and one patient underwent surgical removal. The remaining two patients had relatively minor bleeding and were managed conservatively. All of them had a favorable clinical outcome with a KPS score of 90 after treatment. Within the entire patient population, six patients (26.1%) in the diffuse-type group and 17 patients (13.0%) in the compact-type group underwent repeated GKRS for remnant AVM.
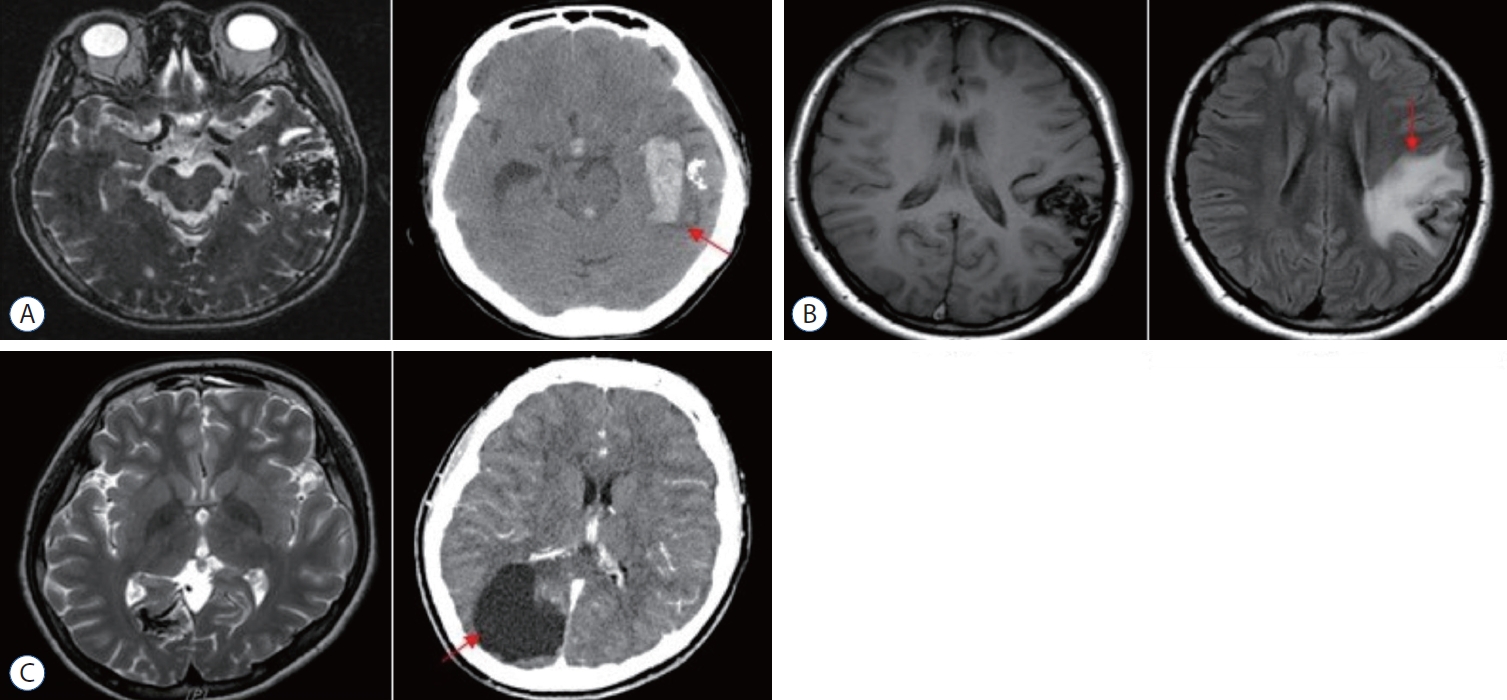
Post-Gamma Knife radiosurgery complications. A : Hemorrhage. Non-enhanced computed tomography (CT) scan shows acute intracranial hemorrhage in the left temporal lobe (red arrow). B : Radiation induced change. In the T1 magnetic resonance imaging image, radiation-induced edematous change is observed around the arteriovenous malformation (AVM) nidus in the left precentral gyrus (red arrow). C : Cystic formation. Hypodense cystic formation are visible around the AVM in the enhanced CT scan (red arrow).
Univariate and multivariate analyses of factors associated with total obliteration
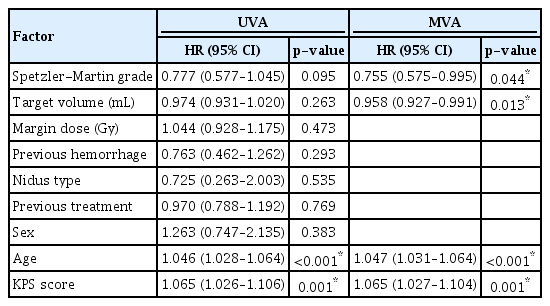
Table 5 shows the results of the univariate and multivariate analyses of factors associated with complete AVM obliteration. In the univariate analysis of all patients with AVM, older age (HR, 1.046; 95% CI, 1.028–1.064; p<0.001) and lower KPS score (HR, 1.065; 95% CI, 1.026–1.106; p=0.001) were both associated with a reduced likelihood of complete obliteration across both groups. Conversely, target volume, marginal dose, previous hemorrhage, nidus type, and history of previous treatment (radiosurgery, resection, or embolization) were not significantly associated with complete obliteration. Among the significant factors identified in univariate analyses, both older age (HR, 1.047; 95% CI, 1.031–1.064; p<0.001) and lower KPS score (HR, 1.065; 95% CI, 1.027–1.104; p=0.001) were significantly associated with a lower likelihood of complete obliteration in the multivariate analysis of all patients with AVM. Unlike the results of the univariate analysis, multivariate testing showed that a high Spetzler-Martin grade (HR, 0.755; 95% CI, 0.575–0.995; p=0.044) and large AVM target volume (HR, 0.958; 95% CI, 0.927–0.991; p=0.013) were significantly associated with decreased chances of complete obliteration.
Case presentation 1
Fourty-two years old man who has known history of hepatitis B was admitted after outpatient at Ajou University Hospital for headache. T2-weighted MRI shows a compact-type AVM in right occipital lobe (Fig. 4A). The DSA confirmed 2.4 ×1.8 cm sized unruptured AVM in right occipital lobe cortical region, with feeding vessels from terminal branch of right anterior cerebral artery, middle cerebral artery, posterior cerebral artery and draining via posterior one third of superior sagittal sinus (Fig. 4B). This patient underwent GKRS (1.8 mL, 20 Gy to 50% of isodense line) (Fig. 4C). The follow-up angiography revealed (41 months after GKRS) complete obliteration of the AVM without any complication (Fig. 4D).
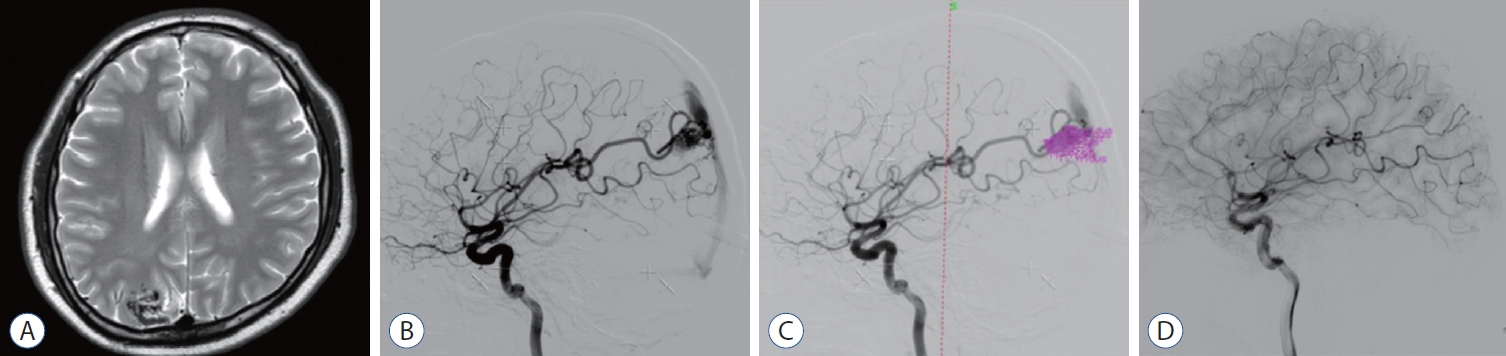
A : T2-weighted magnetic resonance imaging shows an compact-type arteriovenous malformation (AVM). B : Digital subtraction angiography confirmed 2.4×1.8 cm sized AVM in right occipital lobe cortical region, with feeding vessels from terminal branch of right anterior cerebral artery, middle cerebral artery, posterior cerebral artery and draining via posterior one third of superior sagittal sinus. C : Gamma Knife radiosurgery (GKRS) target location is shown (1.8 mL, 20 Gy to 50% of isodense line). D : The follow-up angiography revealed (41 months after GKRS) complete obliteration of the AVM.
Case presentation 2
Fourty-eight years old woman who has no other medication history was admitted after outpatient at Ajou University Hospital for persistent headache and memory loss. T1-weighted enhanced MRI showed a diffusely enhanced AVM in the left posterior parietal lobe (Fig. 5A). The DSA confirmed 4.2×3.8 cm sized unruptured AVM in left posterior parietal lobe cortical region, with feeding vessels from left anterior cerebral artery, middle cerebral artery, posterior cerebral artery and trandural collaterals from middle meningeal artery posterior division draining via cortical vein to superior sagittal sinus and vein of Labbe to sigmoid sinus (Fig. 5B). Spetzler-Martin scale score was 4 (size 3–6 cm, deep drainage, parenchymal eloquence). This patient underwent GKRS (14.1 mL, 14 Gy to 50% of isodense line) (Fig. 5C). The follow-up angiography revealed (34 months after GKRS) complete obliteration of the AVM without any complication (Fig. 5D).
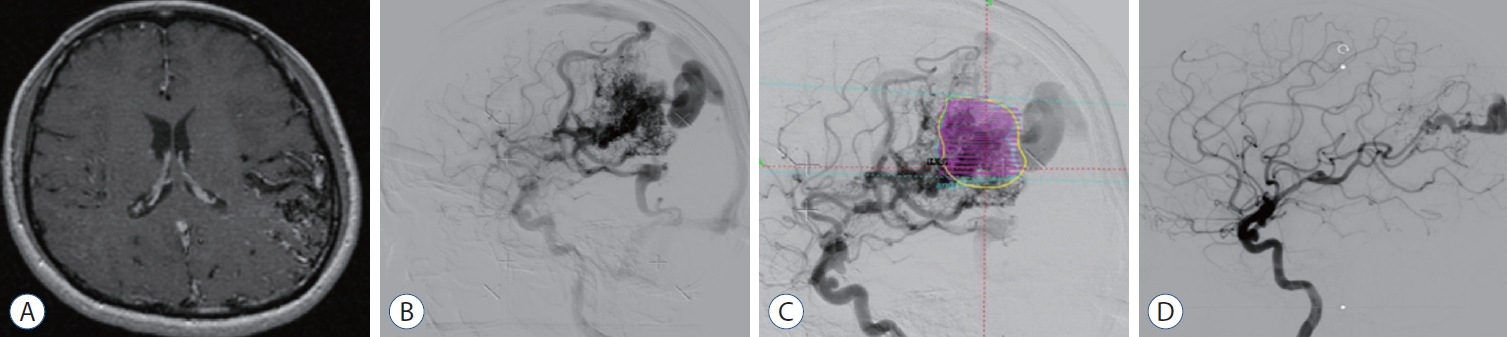
A : T1-weighted enhanced magnetic resonance imaging shows an diffuse-type enhanced arteriovenous malformation (AVM). B : Digital subtraction angiography confirmed 4.2×3.8 cm sized AVM in left posterior parietal lobe cortical region, with feeding vessels from left anterior cerebral artery, middle cerebral artery, posterior cerebral artery and transdural collaterals from middle meningeal artery posterior division draining via cortical vein to superior sagittal sinus and vein of Labbe to sigmoid sinus. C : Gamma Knife Radiosurgery (GKRS) target location is shown (14.1 mL, 14 Gy to 50% of isodense line). D : The follow-up angiography revealed (34 months after GKRS) complete obliteration of AVM.
DISCUSSION
GKRS is an established effective treatment strategy for brain AVM [13,19]. GKRS is preferred to surgical resection, especially when the AVM location is difficult to access or the risk of hemorrhage is high [16,21]. More recently, GKRS has also become the preferred method of intervention for higher grade AVMs (defined by a large AVM size, eloquent location, and deep portion), and GKRS after incomplete microscopic resection is reportedly more beneficial than repeated resection [4]. In the case of diffuse-type nidus AVM, the boundary with the surrounding normal tissue is unclear, resulting in a high complication risk for surgical resection and difficulty completely removing the lesion [6]. Moreover, obliterating the nidus using endovascular embolization is difficult because the extent of the nidus is unclear, the effects are limited, and a residual AVM nidus often remains after endovascular embolization [11]. The effectiveness of GKRS has been demonstrated in several studies [3,5]. However, whether the usefulness and feasibility of GKRS is comparable across nidus types is unknown.
To the best of our knowledge, the present study is the first to conduct an intergroup comparison between nidus types to determine whether GKRS treatment is equally useful and effective across groups. Diffuse-type nidus AVMs were more frequently distributed in eloquent locations compared to compact-type nidus AVMs (p=0.015), mirroring previous findings [6]. Although the underlying cause is unclear, the perforating arteries in eloquent locations are distributed more according to the white matter tract, and diffuse nidi include comparatively more of these perforating arteries [6]. Furthermore, diffuse-type AVMs are larger in terms of size and extent of invasion of adjacent normal brain tissues, increasing the odds of occurrence close to eloquent areas.
Our data showed no significant differences in complications between compact- and diffuse-type AVMs (p=0.133). Post-GKRS RIC were the most common complications, occurring in 13.6% of all cases, followed by bleeding in 2.6% and cyst formation in 0.6% of patients. Substantiating this finding, previous studies also revealed that RIC are the most common post-GKRS complication [7,8]. The cause of RIC is unclear, but it is hypothesized that these changes are the result of blood-brain barrier disruption [12]. Rarely, RIC cause permanent neurologic deficits [7,8]. According to a recently published metaanalysis of GKRS in patients with AVM, RIC were reported in an average of 29.0% of cases [2]. Our study identified a comparatively low RIC complication rate at 13.6%. In the meta-analysis, the average marginal and maximum radiation doses were 20 Gy (range, 19–22) and 37.9 Gy (range, 36–40), respectively, compared with 16.8 Gy and 33.5 Gy in our study. As such, we speculate that the difference in RIC occurred because of the lower radiation dose in our cohort. In our study, of the 21 patients that developed RIC after GKRS, 14 had unruptured AVMs, and the remaining seven AVMs were ruptured. None of the patients developed any permanent neurological deficits. The major risk associated with GKRS is hemorrhage during the latency interval between GKRS and obliteration. The reported risk of bleeding varies from 2.01% to 18.18%, with a median rate of 6.2% [20]. The risk of bleeding in our study (3.6%, 0.90% annually) was slightly lower. In a previous study, the natural hemorrhagic risk of AVMs was reported as approximately 2.2% to 3.0%, which can be considered a meaningful result with GKRS as the primary outcome [10,19].
The complete obliteration rate in all cases was 48.1%. Obliteration was determined by assessing the disappearance of the nidus on DSA and MRI after treatment, an approach commonly used in previous studies [12,15].
Pollock et al. [19] identified a complete obliteration rate of 59.1% at 4 years and 85.1% at 8 years. Furthermore, a rate of 56.7% was observed in the meta-analysis published in 2022 [2,19]. Our comparatively lower result can be attributed to the inclusion of patients with more ruptured AVMs, high AVM Spetzler-Martin grades, shorter follow-up periods, and our strict imaging interpretation criteria. In compact-type nidus AVMs, the complete obliteration rate tended to be higher than that seen with diffuse-type nidus AVMs, but this difference was not statistically significant. Similar results have been reported in previous studies because the Spetzler-Martin grade and AVM size tend to be larger for diffuse-type AVMs, which take a long time to completely obliterate during the follow-up period [6].
Factors previously associated with complete AVM obliteration include patient age at radiosurgery, sex, maximum AVM diameter, AVM volume, angiographically delineated shape of the AVM nidus, number of veins, Spetzler-Martin grade, marginal dose, repeated treatment, and previous hemorrhage [1,20,23,24]. In our study, a high Spetzler-Martin grade (p=0.044), large target volume (p=0.013), old age (p<0.001), and lower KPS score (p=0.001) were factors significantly related to lower complete obliteration rates in the multivariate analysis. In Table 2, the diffuse nidus group showed a significantly larger volume and higher Spetzler-Martin grade than the compact nidus group. There is a possibility that such differences may have influenced our interpretation of the results. Although not statistically significant (p=0.434), the total obliteration rate for the diffuse type was lower at 39.1% compared to the compact nidus group (48.1%). Additionally, the proportion of patients receiving retreatment was higher at 26.1% for the diffuse group compared to the compact group (13.0%). It is believed that these results correspond to these differences. The impact of diffuse-type nidus AVM on GKRS outcomes is controversial; one study reported that diffuse-type nidus was an associated factor in complete obliteration, whereas others, such as ours, have not found statistically significant differences [18,23,24]. However, in the case of diffuse-type nidus AVM, GKRS is usually used with a low radiation dose, and this often comes into effect slowly; hence, it can be generally inferred that complete obliteration can be measured to be low in practice. Considering that the primary purpose of AVM treatment is to minimize complications and reduce the risk of bleeding, GKRS is clearly effective and feasible for the treatment of diffuse-type AVM. It can be anticipated that the smaller size of the nidal vessels in the diffuse-type AVM compared to the compact group may allow for effective nidus obliteration through radiation effects even with a low radiation dose. Due to the characteristic of smaller nidal size, diffuse-type AVMs often have limitations in endovascular embolization, and there are limited alternative treatments besides GKRS. Many cases also pose challenges in selecting the appropriate Gamma plan. Therefore, we believe this study is meaningful in comprehensively confirming the results of GKRS for diffuse-type AVMs, considering the frequent ambiguity in treatment decisions.
Our study had several limitations. First, as this was a single-center retrospective study, the comparative analysis was conducted using a small group of patients. Most of the cases were compact-type nidus AVMs (85.1%), resulting in an imbalance in the number of cases compared with those of the diffuse-type nidus group (14.9%). Second, the patient follow-up period may have been insufficient. There would have been a difference in the interpretation of the results if the follow-up was performed for a longer period after GKRS, as future complete obliteration was likely among patients identified as having partial obliteration. Finally, despite classifying the nidus type based on objective criteria using MRI and DSA, there are some cases where the classification is somewhat ambiguous, which could potentially impact the study results.
Nevertheless, this study confirmed the effectiveness of GKRS across nidus types. To compensate for the selection bias and shortcomings of this study, multi-institutional studies that make use of propensity score matching are needed in the future.
CONCLUSION
GKRS is a safe and feasible treatment for brain AVMs characterized by both diffuse- and compact-type nidi. Furthermore, the optimal treatment plan and radiation dose identified by accurate angioarchitectural classification may minimize GKRS complications in AVM.
Notes
Conflicts of interest
No potential conflicts of interest relevant to this study exist.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : YCL; Data curation : JHK, YCL; Formal analysis : JHK, EHH; Methodology : JHK; Project administration : YCL; Visualization : JHK; Writing - original draft : JHK; Writing - review & editing : EHH, JHS, YCL
Data sharing
None
Preprint
None