Blood-Blister Aneurysms of the Internal Carotid Artery in Tibetan and Han Populations : A Retrospective Observational Study
Article information
Abstract
Objective
Blood-blister aneurysms (BBAs) of the internal carotid artery (ICA) are challenging lesions with high morbidity and mortality rates. Although research on BBAs is well documented in different populations, the study of BBAs in the Tibetan population is extremely rare. This study aimed to evaluate the characteristics of BBAs and analyze the treatment modalities and long-term outcomes in the Tibetan population in comparison with the Han population.
Methods
The characteristics of patients with BBAs of the ICA from January 2009 to January 2021 at our institution were reviewed. The features of aneurysms, treatment modalities, complications, and follow-up outcomes were retrospectively analyzed.
Results
A total of 130 patients (41 Tibetan and 89 Han patients) with BBAs of the ICA who underwent treatment were enrolled. Compared with the Han group, the Tibetan group significantly demonstrated a high ratio of BBAs among ICAs (8.6%, 41/477 vs. 1.6%, 89/5563; p<0.05), a high ratio of vasospasm (34.1%, 14/41 vs. 6.7%, 6/89; p=0.001), a high risk of ischemic events (43.9%, 18/41 vs. 22.5%, 20/89; p<0.05), and a low ratio of good outcomes (modified Rankin scale, 0–2) at the 1-year follow-up (51.2%, 21/41 vs. 74.2%, 66/89; p<0.05). The multivariate regression model showed that ischemic events significantly contributed to the prediction of outcomes at 1 year. Further analysis revealed that microsurgery and vasospasm were associated with ischemic events.
Conclusion
In comparison with Han patients, the Tibetan population had a high ratio of BBA occurrence, a high incidence of ischemic events, and a high ratio of poor outcomes. The endovascular approach showed more benefits in BBA patients.
INTRODUCTION
Blood-blister aneurysms (BBAs) are challenging lesions with high morbidity and mortality and account for 0.9–6.5% of all internal carotid artery (ICA) aneurysms [1,15,27]. BBAs are mostly located on the nonbranching sites of the supraclinoid ICA and commonly have a thin, fragile wall and a poorly defined neck, with a tendency to increase and change in shape [7,15,20]. Considering the characteristics of BBAs, the occurrence rates of intraoperative and postoperative aneurysmal ruptures are higher than those of other types of aneurysms [23].
Many studies have documented intracranial BBAs in different populations; however, studies on intracranial BBAs in the Tibetan population remain limited. In western China, the majority of the Tibetan population lives in the Tibetan Plateau, Garzê, and Aba regions, with an average elevation of >3000 m above sea level. These regions are characterized by low barometric pressure and oxygen-thin air. Tibetans belong to an ethnic group that is characterized by a unique lifestyle of special high salt and high fat and cholesterol diets. These are considered risk factors for hemorrhagic stroke [6].
Although our previous study reported that Tibetan BBA patients are associated with a high risk of occurrence in atypical locations and a high incidence of cerebral infarction with poor prognoses, data on BBAs of the ICA are still lacking [2]. Therefore, the present retrospective study was conducted regarding the treatment of BBAs of the ICA at our institution to compare the characteristics of Tibetan and Han populations and analyze the clinical outcomes and prognostic factors. To the best of our knowledge, this is the largest single-center BBA cohort study with respect to different ethnic groups to date.
MATERIALS AND METHODS
This study was approved by the West China Hospital Ethics Committee. Written informed consent was obtained from all patients or their families.
Patients
This study included patients with BBAs who were admitted to West China Hospital from January 2009 to January 2021. The diagnosis of BBA was defined as aneurysms that arose from the nonbranching site of the supraclinoid ICA based on computed tomography angiography (CTA) and digital subtraction angiography (DSA). Patient data, including patient characteristics, blood testing, Hunt-Hess grades, Fisher grades, treatment methods, initial results, complications, and follow-up outcomes, were collected and analyzed retrospectively.
Preoperative examination and management
All patients were sent to the emergency unit to initiate standard medical treatment and care. Routine blood tests, biochemical examinations, preoperative DSA and 3-dimensional images were performed. The medical history (hypertension, diabetes mellitus, and hypercholesterolemia) and neurologic physical examination were also recorded. Their vital signs were monitored, and supportive treatments were given accordingly. Adequate analgesia, antiemetics, and sedation were given for rapid pain relief. Systolic blood pressure (SBP) was controlled at less than 160 mmHg. Patients with hypertension might benefit from an SBP of 160 mmHg, so relaxed blood pressure control was achieved in these patients (SBP <170 mmHg). Otherwise, if intracranial pressure monitoring is available, then cerebral perfusion pressure greater than 60 mmHg should be maintained.
Treatment
Based on the interdisciplinary consensus, endovascular or surgical treatment modality for the aneurysms was selected. In craniotomy, we often choose delayed elective surgery. Surgery was performed by senior authors under general anesthesia. The pterional approach was used for all patients with BBAs of the ICA. Depending on the operating surgeon’s decision, various surgical techniques were applied, including direct clipping with parallel clipping to the ICA, clipping after wrapping, and trapping with or without bypass. All endovascular procedures were performed under general anesthesia, and the patients were systemically heparinized during the procedure. Neuroform (Stryker, Kalamazoo, MI, USA) and Enterprise stents (Codman, Raynham, MA, USA) and coils were used for endovascular treatment. Subsequently, angiography was repeated to evaluate the outcomes of embolization. Postoperatively, dual antiplatelet medication was given regularly. Triple-H (hypertension, hypervolemia, and hemodilution) therapy was used for all patients.
Follow-up and definitions
Procedure-related complications were documented, and the results of all subsequent angiographies were recorded. The modified Rankin scale (mRS) was used to assess the clinical outcomes during discharge and at the 1-year follow-up. Favorable outcomes were defined as mRS scores of 0–2. Cerebral vasospasm refers to vasoconstriction as confirmed by CTA or DSA. Intraoperative rupture was defined as premature rupture of the aneurysm during the procedure, resulting in considerable hemorrhage. Cerebral infarction was confirmed by postoperative head CT scan.
Statistical analysis
Data were analyzed using SPSS software 22.0 (IBM, Armonk, NY, USA). p<0.05 was considered to be statistically significant. Data are presented as the means±standard deviation for continuous variables or counts (percentages) for categorical variables. Differences between groups were analyzed using t tests, Mann-Whitney U tests, Fisher’s exact tests, or chisquared test as appropriate. Multiple-variable logistic regression models were used to predict the outcomes.
RESULTS
Characteristics of patients
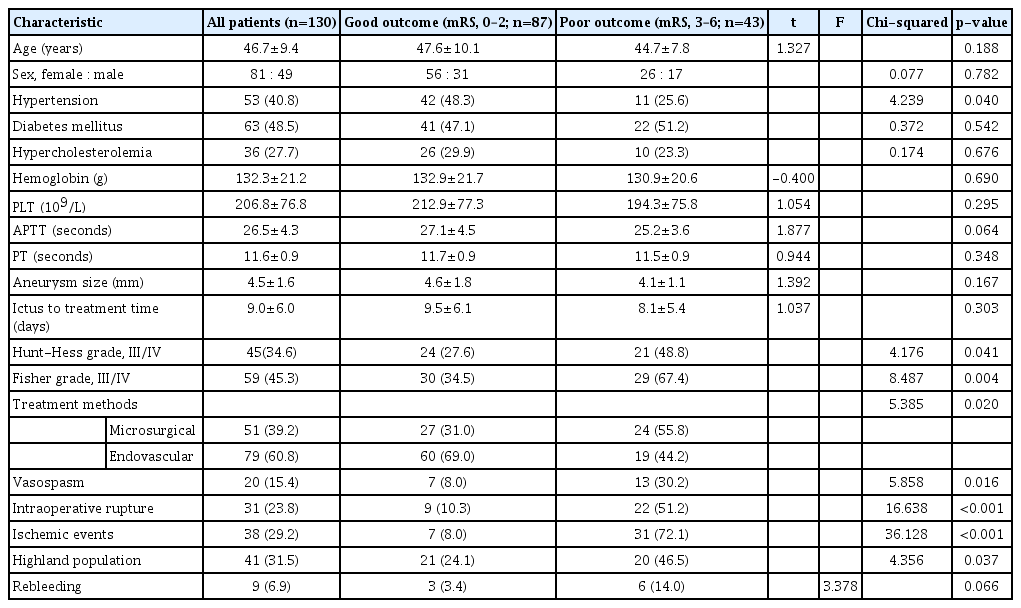
Between 2009 and 2021, 6040 aneurysm patients with subarachnoid hemorrhage (SAH) were admitted to West China Hospital. Of these, 477 patients (7.9%) were Tibetans from the Tibetan Plateau, Garzê, and Aba regions. Forty-one (8.6%) of these Tibetan aneurysmal patients and 89 (1.6%) of 5563 Han aneurysmal patients were diagnosed with BBAs of the supraclinoid ICA, and there was a statistically significant difference between the patients (p<0.05). The mean age of the patients in the two groups was 44.4±8.0 and 47.7±9.9, respectively. The cohorts comprised 81 females and 49 males, with a female/male ratio of 1.65 : 1, and there was a slight female predominance in both groups. A larger number of patients in the Han group had hypertension (44/89, 49.4% vs. 9/41, 22.0%; p=0.018) and diabetes mellitus (51/89, 57.3% vs. 12/41, 29.3%; p=0.016). The patients in the Tibetan group had a significantly higher mean hemoglobin value (141.3±21.0 vs. 128.2±20.2, p=0.007), mean platelets (PLT) value (234.2±85.3 vs. 194.3±70.0, p=0.025), longer mean activated partial thromboplastin time (28.4±5.3 vs. 25.6±3.5, p=0.003), and longer mean time from ictus to treatment (11.3±7.4 vs. 8.0±4.8, p=0.013) than the Han group. Moreover, the occurrence of vasospasm was significantly higher in the Tibetan group than in the Han group (14/41, 34.1% vs. 6/89, 6.7%; p=0.001). The two groups showed no significant differences in gender, hypercholesterolemia, PT, Hunt-Hess grade, Fisher grade, aneurysmal size, multiple aneurysmal number, and treatment method on admission (Table 1).
Treatment information of patients
In this study, 51 patients were treated by microsurgery, which included direct clipping (n=41), clipping after wrapping (n=3), and trapping without bypass (n=7), while 73 patients underwent embolization with stent-assisted coiling, including a single stent in 13 patients, double in 32, and triple in 28. Only four patients were treated with coiling alone due to the narrow neck of the aneurysms. Intraoperative rupture occurred in nine patients in the Tibetan group and 22 patients in the Han group but showed no significant difference. The Tibetan group had a significantly higher ratio of postoperative infarction than the Han group (18/41, 43.9% vs. 20/89, 22.5%; p=0.034). Rebleeding and recurrence occurred in nine and 11 patients in the Han group, respectively, but were not observed in the Tibetan group.
Outcomes
A total of 26 patients (11 patients [26.8%] in the Tibetan group vs. 15 patients [16.9%] in the Han group, p=0.332) died during the perioperative period. In the Tibetan group, nine patients were deceased as a result of postoperative massive cerebral infarction, and two patients died due to intraoperative rupture when undergoing endovascular treatment. In the Han group, nine patients died of postoperative massive cerebral infarction, four patients died of rebleeding, and two patients died of intraoperative rupture. This was followed by the death of seven patients during the 1-year follow-up period, six due to complications after experiencing postoperative mass infarction and one due to recurrence. At the 1-year follow-up, 10 Han patients with poor condition at discharge (mRS, 3–5) achieved further recovery (mRS, 0–2) and had satisfactory outcomes. Such a transition was not available in the Tibetan group. A good grade (mRS, 0–2) was achieved in 51.2% of the Tibetan patients and 74.2% of the Han group patients, with a significant difference (p=0.037, Table 2).
In the present study, univariate analysis indicated that hypertension, Hunt-Hess grade (III/IV), Fisher grade (III/IV), treatment method, vasospasm, intraoperative rupture, ischemic event, and Tibetan group showed significant correlations with prognosis (p<0.05, Table 3). Multivariate logistic regression revealed that ischemic events were an independent risk factor for unfavorable outcomes (odds ratio [OR], 15.72; 95% confidence interval [CI], 2.701–91.497; p=0.002; Table 4). Further analysis showed that microsurgery (OR, 66.788; 95% CI, 6.49–687.275; p<0.001) and vasospasm (OR, 52.89; 95% CI, 4.498–621.943; p=0.002) significantly contributed to ischemic events (Table 5).
DISCUSSION
Ethnic minority regions are mostly located in remote and economically deprived rural areas. There are notable differences in culture, economy and health-care accessibility between ethnic minority regions and other regions of China. The Tibetan ethnic group is characterized by a unique lifestyle of a special local diet of tsampa, butter tea, mutton, and beef, with a high cholesterol and salt diet. Moreover, the majority of Tibetans reside at high altitudes, such as the Tibetan Plateau, Garzê, and Aba regions, at an average elevation of approximately 3000 m above sea level [30]. Due to their unique lifestyle and residential circumstances, the prevalence of cardiovascular disease risk factors, including hypertension, diabetes, overweight or obesity, dyslipidemia, and current smoking, was high in Tibetans [30].
Most intracranial aneurysms typically arise at vascular branch points due to alterations in hemodynamic forces at these points [28]. The histological features of BBAs include focal wall defects covered with clot and fibrous tissue [11]. Currently, the mechanism of BBA formation remains unclear. Previous studies have revealed that several risk factors, including arteriosclerosis, hypertension, hemodynamic changes, and dissection of the cerebral artery, contribute to the pathogenesis of BBAs [11,23]. However, the true pathogenesis is yet to be elucidated. In our study, among 477 Tibetan aneurysmal patients, there were 41 (8.6%) BBA patients, and this ratio was significantly higher than that observed in the Han patient population (89/5563, 1.6%) and in previously reported data [27]. Due to the high ratio of BBAs among Tibetan aneurysmal patients, we speculated that the Tibetan population is more susceptible to BBAs. Further analysis revealed significant differences between the two groups in the baseline information, including red blood cell count, hemoglobin level, and hematocrit level, suggesting that these factors may increase the incidence of BBAs in the Tibetan population. The anteromedial wall of the supraclinoid portion of the ICA is curved as the flux of food flow impinges on the arterial wall [1]. Therefore, hemodynamic stress, which could be influenced by hemoglobin level, seems to be important in the formation of BBA [23].
It is important to evaluate effective treatment strategies for BBAs due to their high incidence of intra- or postoperative bleeding among BBA patients. Direct clips are the most common strategy for application in BBAs of ICA [10,13,16,23,25]. Distal and proximal temporary clips remain crucial for these dangerous lesions, as these clips could prevent rupture, control bleeding, and soften the aneurysms [25]. An angled or slightly curved clip along with blades was applied parallel to the supraclinoid ICA (including the normal arterial wall beyond the lesion), which more or less narrows the ICA. Therefore, postoperative infarction might occur, as in our case series (17/41, 41.4%), when using direct clips. Intraoperative rupture usually occurs during surgical manipulation of these lesions with fragile walls. Clipping after wrapping still remains a debate due to its unclear efficacy. Ogawa et al. [23] demonstrated that wrapping did not prevent rebleeding and was related to a high incidence of postoperative bleeding and death (3/4, 75%). However, Lee et al. [16] conducted a study in 18 BBA patients, 15 of whom underwent clipping and wrapping. The results revealed that no patients had postoperative bleeding, and only two patients with global infarction died. Murai et al. [21] reported that BBA patients who underwent trapping with radial artery grafting indicated a high risk of ischemic complications. Trapping with bypass often requires the acquisition of new blood vessels, which is not suitable for every patient, and the operation is more complicated. Therefore, our center advocates that trapping with bypass is an optimal surgical modality when direct clipping and clipping with wrapping are unavailable. However, among the 51 patients in our center, all of them could be directly clipped or clipped with wrapping after a proper operation. Therefore, our study included a very low rate of trapping with bypass among the treatment modalities.
Various endovascular strategies, including coil embolization, stent-assisted coiling, and flow-diverting stents, have been used to treat BBAs [17,26,33]. Single coil embolization is potentially hazardous and cannot be considered for BBA treatment due to the shallow sac and wide fragile neck [32]. The stent can provide a bridge by encompassing the BBA neck with vascular endothelial cells. Lim et al. [17] included 34 patients with ruptured BBAs in their study, and 25 of these patients had favorable outcomes. In our study, we used a single stent or overlapping stents with coiling in 49 patients and coiling alone in three patients. Although high morbidity and mortality occur with endovascular therapy, it is associated with more favorable outcomes than microsurgery treatment.
Tibetan BBA patients had a higher ratio of postoperative ischemic events than the Han group. Moreover, univariate analysis revealed that Tibetan group, Hunt-Hess grade, Fisher grade, treatment method, and vasospasm contributed to postoperative ischemic events, which are considered single risk factors by multivariate analysis for the outcomes of BBAs. According to the results of this research, we speculated that a high incidence of postoperative ischemic events was considered one of the most common features for primary hemorrhagic neurovascular diseases in the Tibetan populace.
Currently, the natural history of BBA is uncertain [26], and small sample sizes and substantial study heterogeneity make strong conclusions difficult [22], so the optimal timing of treatment for BBA is still uncertain. A study compared the impact of different surgical timings on patient prognosis, and the results showed that there was no significant difference between early surgery and delayed elective surgery [5]. However, in the acute stage of the disease, the brain tissue is swollen, and the operation is difficult and risky, so we often choose delayed elective surgery. In the future, larger-scale and multitime randomized controlled studies are needed to determine the optimal timing of treatment for ruptured aneurysms.
One of the most obvious differences between the Tibetan and Han populations was the hemoglobin concentration, which was influenced by hypoxia in the high-altitude atmosphere. High-level hemoglobin is widely considered a risk factor for high blood viscosity [9,18,19]. Some studies have demonstrated that the mechanism of high blood viscosity contributes to cerebral ischemic events based on tiny thrombosis formation in the cerebral circulation system [8,12,24]. The other factor that contributes to postoperative ischemic events in the Tibetan population might be PLT. Several studies have concluded that PLT count was positively correlated with the risk of ischemic stroke [4,29]. Therefore, hemoglobin concentration and PLT count might be considered two essential parameters for the development of ischemic events in the Tibetan population.
Otherwise, regarding the potential mechanisms that are responsible for the high incidence of ischemic events, some studies have indicated that vasospasm after SAH, which is more obvious in the Tibetan population in our study, is considered the most important factor [3,14,31], which is consistent with our study findings. The other risk factor for ischemic events was microsurgery treatment, which meant that the endovascular approach was more effective and safer than surgical clipping.
CONCLUSION
This study aimed to explore the difference in BBAs between Tibetan and Han populations. Our research revealed that BBAs in the Tibetan population demonstrated a high risk of occurrence, high ratio of vasospasm, high incidence of ischemic events, and high ratio of poor prognostic rates at the 1-year follow-up. Endovascular treatment remained more beneficial for BBA patients when compared with the high ratio of ischemic events achieved by microsurgery. The other main risk factors for ischemic events included high hemoglobin concentration, high PLT count, and vasospasm in the Tibetan population.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Author contributions
Conceptualization : YR, JL; Data curation : HL, AX, LL, HS, YL, HL, LM, CWZ, CHW, MH, CY; Formal analysis : BH; Funding acquisition : YZ, YR; Methodology : BH, YR; Project administration : YR; Visualization : BH, YR; Writing - original draft : BH; Writing - review & editing : YR, JL
Data sharing
None
Preprint
None
Acknowledgements
Sichuan Province Science and Technology Support Program (CN). 2019YFS0397; Natural Science Foundation of China. 81571131.