Neurosurgical Management of Cerebrospinal Tumors in the Era of Artificial Intelligence : A Scoping Review
Article information
Abstract
Central nervous system tumors are identified as tumors of the brain and spinal cord. The associated morbidity and mortality of cerebrospinal tumors are disproportionately high compared to other malignancies. While minimally invasive techniques have initiated a revolution in neurosurgery, artificial intelligence (AI) is expediting it. Our study aims to analyze AI’s role in the neurosurgical management of cerebrospinal tumors. We conducted a scoping review using the Arksey and O’Malley framework. Upon screening, data extraction and analysis were focused on exploring all potential implications of AI, classification of these implications in the management of cerebrospinal tumors. AI has enhanced the precision of diagnosis of these tumors, enables surgeons to excise the tumor margins completely, thereby reducing the risk of recurrence, and helps to make a more accurate prediction of the patient’s prognosis than the conventional methods. AI also offers real-time training to neurosurgeons using virtual and 3D simulation, thereby increasing their confidence and skills during procedures. In addition, robotics is integrated into neurosurgery and identified to increase patient outcomes by making surgery less invasive. AI, including machine learning, is rigorously considered for its applications in the neurosurgical management of cerebrospinal tumors. This field requires further research focused on areas clinically essential in improving the outcome that is also economically feasible for clinical use. The authors suggest that data analysts and neurosurgeons collaborate to explore the full potential of AI.
INTRODUCTION
Artificial intelligence (AI) is defined by Zini [61] as “AI is a computer-based science which aims to simulate human brain faculties using a computational system.” Hashimoto et al. [18] describes four main branches or subtypes of AI, including machine learning, artificial neural networks, natural language processing, and computer vision. Machine learning is an application or subset of AI in which machines can learn from the data without any prominent programming [18]. The AI can process vast amounts of medical information that can aid in diagnosing and managing neurological conditions [61]. Albeit, AI has demonstrated numerous benefits in the neurological care of patients with cerebrospinal tumors, although certain disadvantages have been recognized. Senders et al. [48] note that though AI can enhance certain aspects of clinicians’ duties, it requires a substantial amount of quality dataset to generate AI models that are viable and deployable in the neurosurgical care of patients. Racine et al. [45] identify that some of the challenges pertain to the nature of the informed consent needed, transparency and ownership of patient data, privacy and discrimination. They propose that overcoming these ethical challenges will help integrate AI into patient care [45]. Furthermore, in a cross-sectional survey, Palmisciano et al. [42] found that more than 75% of the participants wanted neurosurgeons to remain in charge of the provided care ultimately. Hence, Senders et al. [49] mentioned that our perspective for AI in neurosurgery should be to understand how AI and man can perform together instead of AI versus man.
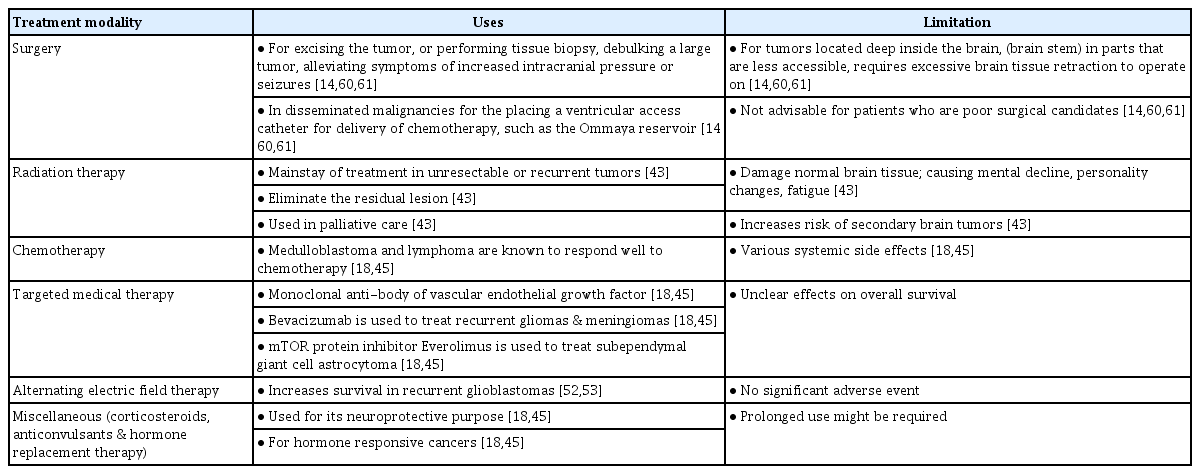
To better understand AI and the impact of its development in the neurological care of patients with cerebrospinal tumors, it is vital to understand the management and prognosis of cerebrospinal tumors prior to the era of AI. Cerebrospinal tumor management is a multi-modality approach, depending on tumor type, grading, location, as well as individual patient characteristics. Treatment can be either monotherapy or a combination of surgical resection, radiation therapy, chemotherapy, newer biological agents, or even alternating electric field therapy (Table 1) [39].
The prognosis of CNS tumors depends on the type of tumor, malignant or benign, and the patient’s age [14,39,46,55,56]. According to the data published by the American Cancer Society, the 5-year relative survival rate can be as low as <10% in middle-aged patients with glioblastoma, a malignant type of CNS tumor. However, a favorable prognosis is seen in younger patients (20–44 years of age) and those with benign tumors such as meningiomas (84%), oligodendrogliomas (90%), and ependymomas (92%) [3]. In the hope of improving survival, neurosurgeons are constantly incorporating updated scientific data and technological advances into their practice [45]. Therefore, AI is introduced due to the difficulty of treating CNS tumors.
Our study aims to analyze the role AI is currently playing in the neurosurgical management of cerebrospinal tumors. The authors also suggest ways to improve it for a broader role.
MATERIALS AND METHODS
This scoping review was conducted according to the Arksey and O’Malley framework [4]. Our research was guided by the question, “What are the effective ways in which AI can be used in the neurosurgical management of cerebrospinal tumors?” We searched PubMed, Ovid, Web of Science, the Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, EMBASE, and Scopus from January 10 to April 10, 2021. No filters were added. Details regarding study selection are provided using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram in Fig. 1.
Studies focusing on the application of AI in the neurosurgical management of cerebrospinal tumors in human subjects were selected. We included case studies, case series, cross-sectional studies, case-control studies, cohort studies, and review articles. Only articles published in English were considered.
We excluded articles published as grey literature, preprints, and those published in languages other than English. We also excluded records for which we could not gain access to the full text, letters to the editor, meeting reports, systematic reviews, cadaver and animal studies, and articles referring to the application of AI for the neurosurgical management of conditions other than tumors.
The screening was performed in two stages. In the first stage, we screened by title and abstract, and in the second, we reviewed the full-text articles. Screening and tabulation were performed by all authors and recorded in an Excel sheet. Due to the heterogeneity of the topic and vast study types, the authors did not perform a formal quality assessment consistent with scoping review methodology.
RESULTS
The initial database search retrieved 1521 articles and, after removing the duplicates and articles not relevant to our research question, 361 unique articles were identified. After screening the articles in two stages, 110 studies were selected for further analysis. The processes and results are further elaborated in the PRISMA 2020 flow chart (Fig. 1). The application of AI in the neurosurgical management of cerebrospinal tumors, as identified in our review, are presented in tabular form (Table 2). The application of AI from the extracted articles is summarized in the subheadings below.
Preoperative diagnosis
The role of magnetic resonance imaging (MRI) as a diagnostic tool has been pivotal in the modern practice of neurosurgery [13]. This tool allowed surgeons to evaluate structures at the base of the skull and increased the diagnostic accuracy of neuroimaging compared to conventional computed tomography (CT) [35]. The shortcomings of MRI include the difficulty in detecting small metastases and differentiating between tumors, between tumor and infectious foci, as well as between tumor recurrence and treatment effects. Researchers have been trying to address these challenges, and the incorporation of AI might help in that direction [44].
One study demonstrated the use of MRI in a subtype of brain tumor called meningeal tumors and mentioned the importance of AI in its management. Although MRI sequences can aid in identifying the different types of meningeal tumors, these characteristics can overlap with other conditions, thereby adding to the confusion. AI algorithms are effective in detecting these minute differences for accurate diagnosis [28,60]. In a study by Krivoshapkin et al. [28], MRI histogram peaks were used to formulate the AI algorithm to study the tumor volumes. By Tukey test and the Games-Howell test, the authors identified the mean deviation in agreement index between specialists (neurosurgeons and radiologists) was 0.98 (standard error of mean [SEM], 0.007). They then concluded that the advanced algorithm had proved high specificity, sensitivity, and inter-operator repeatability. In another study, the investigators utilized texture analysis algorithms and AI methods to differentiate between benign and malignant tumors, necrosis as a treatment effect, and disease progression, while some have also developed algorithms for calculating the number of metastatic lesions [41]. Researchers have utilized the radiologic dataset to distinguish between skull base chordomas versus chondrosarcomas and predict the molecular subgroups of medulloblastoma in a noninvasive manner [6,11,31,59]. Hu et al. [21] utilized AI techniques to distinguish between high-grade and low-grade tumors. The vastness of possibilities in surgical care with the use of AI is additionally witnessed in the study findings of Ker et al. [26]. They utilized AI to classify glioma pathology specimens and extrapolated this knowledge to breast cancer specimens. Last but not least, Arle et al. [5] found that 95% of posterior fossa tumors in the pediatric population can be accurately diagnosed with the use of AI, whereas only 72% can be predicted accurately by neuroradiologists.
In their study, Senders et al. [48] concluded that it is essential to validate AI algorithms before implementing them clinically thoroughly. They emphasize the importance of incorporating the practical and ethical aspects while developing these algorithms to “bridge the gap between research and clinical care” [49].
Surgical planning
Gross total resection of the tumor is still considered an essential component of managing cerebrospinal tumors. The traditional means to prepare for surgery is limited since it relies heavily on the surgeon’s spatial imagination, practical clinical experience, and understanding of the patient’s condition [51]. For tumors located at the base of the skull or close to the grey-white matter junction, a precise understanding of the anatomy is a prerequisite to perform surgery safely and successfully. Shen et al. [51] propose using a 3D printed model of the skull to understand better the anatomy of the skull base in preparation for endoscopic endonasal transsphenoidal pituitary surgery. Different researchers have utilized AI to better delineate the individual patient’s anatomy for preoperatively preparing the surgeon as to what to expect during surgical navigation. They have found it to be especially useful in skull base surgery, transsphenoidal pituitary surgery, or malignant tumor resection [1,41,57].
Even small angles of a shift in the tissue during surgery can lead to disproportionately adverse outcomes in neurosurgery. Positioning, fixing the head, and identifying landmarks are essential for neuronavigation. Intraoperative brain shifts pose a significant challenge on surgeons while resecting tumors in delicate areas like the base of the skull or surgery of posterior fossa tumors. Frisken et al. [17] proposed the use of a complex finite element method, a method that takes into account biophysics and geometrics to predict shifts accurately in the manual landmarks.
Carlson and Link [9] note that the lifetime prevalence of vestibular schwannoma is one in 500 persons. They are best evaluated by a volumetric measurement and managed based on tumor size. Semi-automated segmentation of tumors, especially schwannomas, saves time and improves segmentation accuracy and effort [9]. However, some limitations were noted regarding semi-automated segmentation that included unpredictability and error.
One of the preferred treatment options for spinal tumors is resection with stabilization. This accurate assessment of bone mineral density is a necessity. Nam et al. [38] have applied machine learning regression algorithms to predict the T-score of vertebrae. The authors found a classifying accuracy of 92.5% in the test data set of 40 vertebrae. They conclude that this algorithm could help the surgeons to plan preoperatively [38].
Lee et al. [30] proposed a study in which generative adversarial networks (GANs) were used to compare image synthesis. GANs are an emerging AI-based technique involving a pair of networks working against each other. This study applied GANs to image synthesis, demonstrating that synthetic systems could be trained using paired data to make MRT2 images from CT scans. The synthesized MRT2 images were further analyzed quantitatively compared to the reference MRT2 images that demonstrated close approximations. This study further concluded that the purpose of using GANs to synthesize MR images from spine CT images would demonstrate better diagnostic usefulness of CT [30].
Virtual training and simulation
Birkmeyer et al. [8] noted in their study that for most surgeries, the patient outcome is better if a surgeon with greater cumulative experience operates on them. Dewan et al. [12] highlight that the neurosurgeon to neurosurgical cases ratio is low, especially in low, middle-income countries. Given this shortage and the adoption of “healthcare for all” as a sustainable developmental goal, they re-emphasize the importance of ensuring that residents receive sufficient, organized skill-oriented training that will enable them to perform surgery confidently even early in their career [12]. AI can be used to train, hone learned surgical skills, and assess neurosurgical residents and early- mid-career surgeons. Different researchers have utilized AI in various aspects of neurosurgical training, such as to improve diagnosis and 3D simulation labs to decrease surgeons’ hand tremors [53,58]. During microsurgery, tremors lead to an increased angle of resection, thereby increasing complications. Decreasing tremors has been shown to reduce the complications of high-risk surgeries directly. 3D simulation labs have also been shown to increase the surgeon’s confidence in performing the surgery, which is especially important for early career surgeons, and in contexts where there is less systematic hands-on clinical experience [10,47,53].
Tumor margin detection
Remnants of tumorous tissue are the leading cause of tumor recurrence. There has been a revolution in neurosurgery with the advent of microsurgery and frozen section histopathological analysis. Despite the progress, the exact intraoperative identification of tumor margins is still an immense challenge faced by surgeons. Manni et al. [33] designed a system utilizing AI to identify tumor margins by hyperspectral imaging with 80% accuracy.
A study conducted by Khalsa et al. [27] reported the use of a microscopic technique called Raman scattering, coupled with an imaging technique called Stimulated Raman Histology to create virtual Hematoxylin and Eosin slides. This AI application helped determine the grade of the tumor without processing the tissue in real-time [27]. The same study reported the intraoperative use of AI algorithms to diagnose, classify and grade pediatric brain tumors with 100% accuracy. In their study, Khalsa et al. [27] noted that a deep convolutional neural network (CNN) was used to distinguish 13 different brain tumors using intraoperative SRH specimens. This CNN-based approach was proven to have a diagnostic accuracy of 94.6%. One of the best results was noted with a binary classifier and support vector machine (SVM) with F1 scores 0.92–0.94. The authors note that human neuro pathologists make diagnoses considering the clinical presentation, imaging findings, and other factors; hence, they predict that automated classifiers will not replace human pathologists, but it will improve the speed and accuracy with which a preliminary diagnosis is made [27].
Survival prediction
Knowing the prognosis for a patient at the time of diagnosis can assist the neurosurgical team in charting the management plan for the patient. In one study, the researchers used AI algorithms to filter prognostic- related genes in high-grade gliomas (HGG) [52]. They noticed that IDH1 mutation status and protein expression of WEE1 were proven to induce HGG cell migration in vitro. They proposed the use of WEE1 as a prognostic biomarker that had potential diagnostic value for HGG patients [52].
Emblem et al. [15] used a SVM and a whole- tumor cerebral blood volume for this purpose, while Nematollahi et al. [40] proposed the C5.0 decision tree model, with tumor width and Karnofsky performance status scores being the most critical parameters for prediction of survival.
Reliable methods to predict the survival rates post-surgery help understand the early postoperative complications (EPC) and the long-term prognosis in such patients. A prospective study conducted by Van Niftrik et al. [57] included data of 688 patients undergoing intracranial tumor surgery at a tertiary hospital between 2015 through 2017. They were assigned a score called the Clavien-Dindo classification score, which is based on histology, anatomical localization, and surgical access. AI algorithms were then used to predict the EPC, which was found to have better predictability than the conventional methods.
Karhade et al. [25] investigated the use of four machine learning (ML) algorithms to predict 30-day mortality in patients undergoing spinal surgery for metastatic disease. Many preoperative factors were used to deduce the algorithms, such as albumin, white blood cell count, hematocrit, alkaline phosphatase, spinal location of the metastasis, and severity of comorbid conditions. The performance of the algorithms was assessed and is now available as an open-access web application [25]. In another study done by Karhade et al. [24], algorithms for the 90-day and 1-year mortality in patients with spinal metastasis were proposed. As the proficiency in the use of AI grows, one can expect that they will be further utilized to increase the accuracy and time efficiency of the desired results.
Robotics
While many are exploring the incorporation of robotics into neurosurgery, it is still in the very nascent stages. Kwoh et al. [29] first described the use of a robotic device (programmable universal machine for assembly) for CT-guided stereotactic biopsy of an intracerebral tumor. Mattei et al. [36] explain how neurosurgeons from a remote workstation used an MRI-compatible robotic arm to successfully perform neurosurgical procedures, such as tumor biopsies and microsurgical dissection. McGrath et al. [37] in their study noted that integration of robotics might aid in improving patient outcomes, mainly by making surgery less invasive.
DISCUSSION
The application of AI in the neurosurgical management of cerebrospinal tumors is a rapidly evolving field. The potential benefits of amalgamating this field AI with current practice are extensive. It ranges from the training of budding neurosurgeons to robotic surgery applications to enhance precision in the diagnosis, pathological interpretation, and postoperative survival prediction.
Summary of the content
Over the past few decades, neuroimaging has advanced by giant strides. However, the subjective nature of the interpretation has posed limitations in making accurate preoperative diagnoses and planning surgery for patients with cerebrospinal tumors. Various authors mention that by deploying computer algorithms, the accuracy of diagnosis and inter-operator repeatability can be improved [28,60]. While many factors make the neurosurgical procedure successful, the surgeon’s planning and competency are integral. AI algorithms have proven helpful in these aspects by improving anatomical delineation and enriching simulation-based training [5,12,36]. The CNN-based approach has proven to increase neuropathologists’ speed and accuracy to make intraoperative tissue diagnoses [26]. This helps the neurosurgeons provide optimal surgical management in total tumor excision and pediatric tumor excision. Robotics are also being explored to improve surgical outcomes, especially by making these surgeries less invasive [24,28,34].
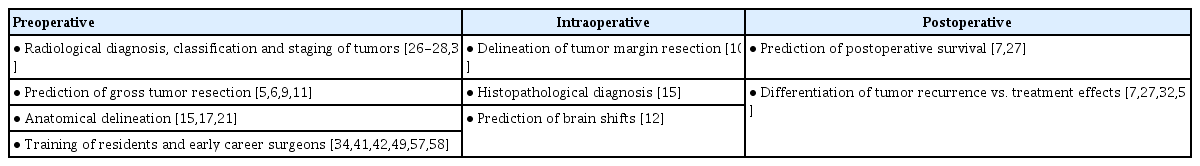
Predicting postoperative survival helps to plan postoperative care for patients with cerebrospinal tumors. Using various algorithms and scores, many authors have devised means to predict early, late postoperative survival for patients with cerebrospinal tumors [23,25,36,51,56]. Pertinent points of AI identified in our analysis have been tabulated into pre-operative, intra-operative and post-operative applications for better visualization and can be seen in Table 3.
Limitations of study design
Potential limitations of this study include the systematic hand search in the databases and the inclusion of only peer-reviewed journal publications written in English and provided either open or institutional access to the full text. Articles in other languages, preprints, unpublished data, or other grey literature were not considered. The authors also did not perform a formal quality appraisal of the selected studies due to the significant heterogeneity.
The reliability of the AI algorithms developed is directly proportional to the size of the dataset utilized to develop it. There are very stringent rules and regulations on using confidential patient data, limiting the amount of data available for the researchers, and developing AI algorithms. Furthermore, these rules are also different in various parts of the world. The research in this field also calls for plenty of monetary input and expertise [19].
Developing uniform health policies worldwide will help scientists further research in this field. Promoting funding and developing data scientists with expertise in healthcare will help overcome various limitations of using AI in the neurosurgical management of cerebrospinal tumors.
CONCLUSION
AI is rapidly evolving, and its applications in cerebrospinal tumor management are rigorously studied. Validation processes and extensive quality assessment of the proposed methods may be necessary to safely and effectively incorporate AI in neurosurgical care. In that direction, an overseeing body could guide decision-making. This field certainly requires further research, primarily focused on the economic and practical feasibility and the resolution of ethical concerns. The constant collaboration of data analysts and neurosurgeons should be encouraged to explore the full potential of AI in the management of tumors and beyond.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : KA; Data curation : KA, AD, SST, AM, SZ; Formal analysis : KA, AD, SST, AM, SZ; Funding acquisition : KA, AD, SZ, ST; Methodology : KA, AD; Project administration : KA, AD; Visualization : KA, AD, SST; Writing - original draft : KA, AD, AM; Writing - review & editing : KA, SZ, AD
Data sharing
None
Preprint
None
Acknowledgements
Previous presentation : the abstract of this manuscript was previously accepted for presentation at: 2021 AANS Annual Scientific Meeting, August 21-25, 2021, Orlando, Florida, USA. E-poster.