Stereoelectroencephalography in Pediatric Epilepsy Surgery
Article information
Abstract
Stereoelectroencephalography (SEEG) is an invasive technique used during the surgical management of medically refractory epilepsy. The utility of SEEG rests in its ability to survey the three-dimensional organization of the epileptogenic zone as well as nearby eloquent cortices. Once concentrated to specialized centers in Europe and Canada, the SEEG methodology has gained worldwide popularity due to its favorable morbidity profile, superior coverage of deep structures, and ability to perform multilobar explorations without the need for craniotomy. This rapid shift in practice represents both a challenge and an opportunity for pediatric neurosurgeons familiar with the subdural grid approach. The purpose of this review is to discuss the indications, technique, and safety of long-term SEEG monitoring in children. In addition to reviewing the conceptual and technical points of the diagnostic evaluation, attention will also be given to SEEG-based interventions (e.g., radiofrequency thermo-coagulation).
INTRODUCTION
Resective epilepsy surgery is the most effective treatment for controlling seizures and improving quality of life among children with medically refractory epilepsy [5,32,34,79,83,95]. The critical step towards achieving favorable surgical outcomes is the accurate delineation of the epileptogenic zone (EZ), or the “minimum amount of cortex that must be resected to produce seizure freedom” [65]. Conceptually, the EZ constitutes a network of structures involved in the generation and propagation of seizures [3]. The goal of epilepsy surgery is to disrupt this network through resection, disconnection, targeted ablation, neurostimulation, or some combination thereof. In some cases, non-invasive evaluation is sufficient to determine the surgical plan, but children frequently require invasive exploration with intracranial electrodes to achieve high-resolution delineation of the EZ and nearby eloquent cortices [33].
Stereoelectroencephalography (SEEG) is an invasive technique used to localize the EZ prior to epilepsy surgery. Pioneered in the 1950s by the French neurosurgeon Jean Talairach and neurologist Jean Bancaud [80,81], the SEEG methodology involves stereotactic implantation of multi-contact intracerebral electrodes for three-dimensional sampling of the EZ. The goal of the SEEG exploration is to establish a spatiotemporal correlation between electrical events in the brain and the patient’s clinical semiology (i.e., the ‘anatomo-electro-clinical’ correlation) [46]. Once concentrated to specialized European centers, SEEG has achieved worldwide usage as a safe and less invasive alternative to monitoring with subdural grid (SDG) electrodes.
This widespread shift in practice has ushered in a new era of SEEG literature, with many studies focused on the pediatric population [1,20,42,44,52,59]. The purpose of this review is to discuss the indications, goals, and technical aspects of invasive SEEG exploration in children. Special attention is given to literature assessing the safety and efficacy of long-term SEEG monitoring. Finally, we briefly discuss the expanding role of SEEG in minimally-invasive epilepsy surgery.
INDICATIONS
Non-invasive evaluation
All epilepsy surgery candidates undergo a routine series of non-invasive tests to formulate an initial anatomo-electroclinical hypothesis. According to the International League Against Epilepsy (ILAE) Subcommission for Pediatric Epilepsy Surgery [26], mandatory components of the non-invasive evaluation include : 1) clinical assessment of epilepsy history, neurological examination, and semiology; 2) interictal scalp EEG recordings (video-EEG capturing ictal events strongly recommended); and 3) structural magnetic resonance imaging (MRI) with epilepsy protocols.
Complementary modalities providing structural, functional, and metabolic data include neuropsychiatric evaluation, computed tomography (CT), magnetoencephalography (MEG), functional MRI, transcranial magnetic stimulation, ictal single photon emission computed tomography (SPECT), positron emission tomography, and other specialized exams (e.g., Wada test). The contributions of these ancillary methods to the overall surgical plan are often difficult to define [17,75]. In a practice survey of 20 pediatric centers [50], approximately 2/3 children who proceeded to resection required non-invasive evaluation only, commonly in the setting of an MRI lesion with concordant semiology and scalp electrophysiology [36].
Invasive monitoring with SEEG
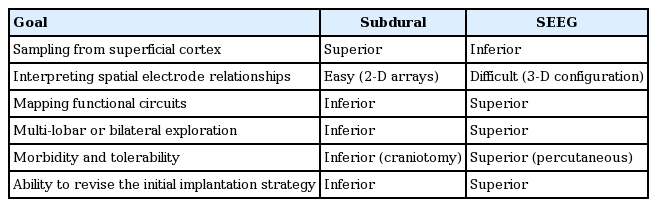
Invasive monitoring is the standard approach for localizing the EZ when non-invasive methods are inconclusive [60]. The two main invasive techniques for long-term monitoring are subdural grids and SEEG. Both methodologies have distinct strengths and weaknesses (Table 1). Subdural electrodes are placed directly on the cortical surface via craniotomy and provide excellent coverage of superficial cortex. Mapping the onset and spread of seizures across the cortical surface is made intuitive by the contiguous arrangement of electrodes, and the high-density coverage of key eloquent cortices (e.g., motor cortex) allows for extensive functional testing with cortical stimulation [72]. SEEG is the preferred modality when the noninvasive evaluation necessitates further evaluation of medial or deep structures (Table 1). This includes any medial cortex, depths of sulci, the insula, and mesial temporal lobe. Percutaneous electrode insertion has a favorable morbidity and tolerability profile relative to craniotomy for SDG placement (see “SAFETY, ACCURACY, AND EFFICACY”). This improved safety and tolerability is particularly evident when widespread or bi-hemispheric coverage is desired. Revising the initial implantation strategy by removing and adding SEEG electrodes is also considerably safer than repositioning SDGs through reopening or expanding the original craniotomy [58]. Finally, SEEG is frequently able to localize the EZ when subdural monitoring has failed [91], thereby availing otherwise inoperable patients of the well-known benefits of epilepsy surgery [34,94].
No consensus guidelines exist for adjudicating between SEEG, grids, or a combination of surface and depth electrodes when planning the invasive strategy. Beyond the general rationale for invasive monitoring, the following indications favor SEEG over subdural recording [20,44,54,55] : 1) hypothesized involvement of deep structures; 2) widespread, multi-focal, or unclear localization of hypothesized ictal onset zone; 3) previous unsuccessful investigation with subdural electrodes; 4) absence of a lesion on MRI imaging; and 5) proposed minimally-invasive treatment strategy (see “THERAPEUTIC APPLICATIONS”).
TECHNICAL PROCEDURES
Preparation
The technique for performing SEEG monitoring in children has been described elsewhere [2,20,42]. Briefly, the methodology begins with non-invasive evaluation and the formulation of an initial anatomo-electro-clinical hypothesis. All patients requiring invasive exploration are discussed at multi-disciplinary conference, where an implantation strategy is devised targeting the presumed seizure onset zones, the propagation territory, relevant eloquent regions, and any structural or functional lesions (e.g., as seen on MEG or SPECT). Sentinel electrodes probing remote cortices may be included to “ruleout” areas of lingering suspicion. Additional electrodes may be added to help define safe resection limits. The goal of exhaustive coverage must be weighed against the small but additive risk conferred by each intracerebral electrode. Strategies vary among institutions, but larger implantations are often 13–15 electrodes [17]. Routine neuroimaging is performed to facilitate trajectory planning and neuro-navigation. At our institution, the imaging protocol consists of high-resolution (3 Tesla) gadolinium-enhanced MRI and thin-slice volumetric CT with contrast. Other groups variably use CT angiography or conventional angiography as well. Regardless of the stereotactic approach, the basic goals are to plan structural targets and avoid major blood vessels with the electrodes. Once desired targets are identified, electrode trajectories can be planned in computer-assisted fashion [76] to maximize the number of desired structures monitored with as few electrodes as possible.
Electrode placement
After the implantation strategy is determined, the neurosurgical team will meet with the patient and family to discuss the rationale for invasive monitoring, procedural details, monitoring plans, and risks/benefits before scheduling a surgical date. Intraoperatively, the patient is administered general anesthesia via endotracheal tube. Electrode placement can be performed using frame-based, frameless stereotactic, or robotassisted methods [92]. At our institution, the Robotic Stereotactic Assistant (Zimmer Biomet, Warsaw, IN, USA) system is used for robot-assisted electrode placement. As is the case for any stereotactic procedure, accurate registration of pre-operative imaging to the patient’s surface anatomy is essential. Registration procedures vary based on the implantation method but must be scrutinized carefully prior to proceeding. After registration is accepted and equipment is calibrated, the planned trajectories are mapped to ensure the robot or surgeon will be able to align each electrode trajectory without colliding with the patient, head mount, or other electrodes. Electrode implantation is comprised of a sequence of steps (Fig. 1) : 1) alignment and verification of trajectory; 2) stab incision with scalpel blade; 3) drilling of small (2–3 mm) burr hole; 4) durotomy with insulated probe and cautery; 5) placement of anchor bolt into burr hole; 6) measurement of electrode length required to achieve target depth (i.e., distance from top of anchor bolt to target); 7) calculating the error between planned electrode length and actual length. High errors require careful re-assessment before the electrode is passed intracranially; 8) placement of electrode through anchor bolt to target depth; 9) securing electrode to anchor bolt; and 10) proceeding to the next trajectory.
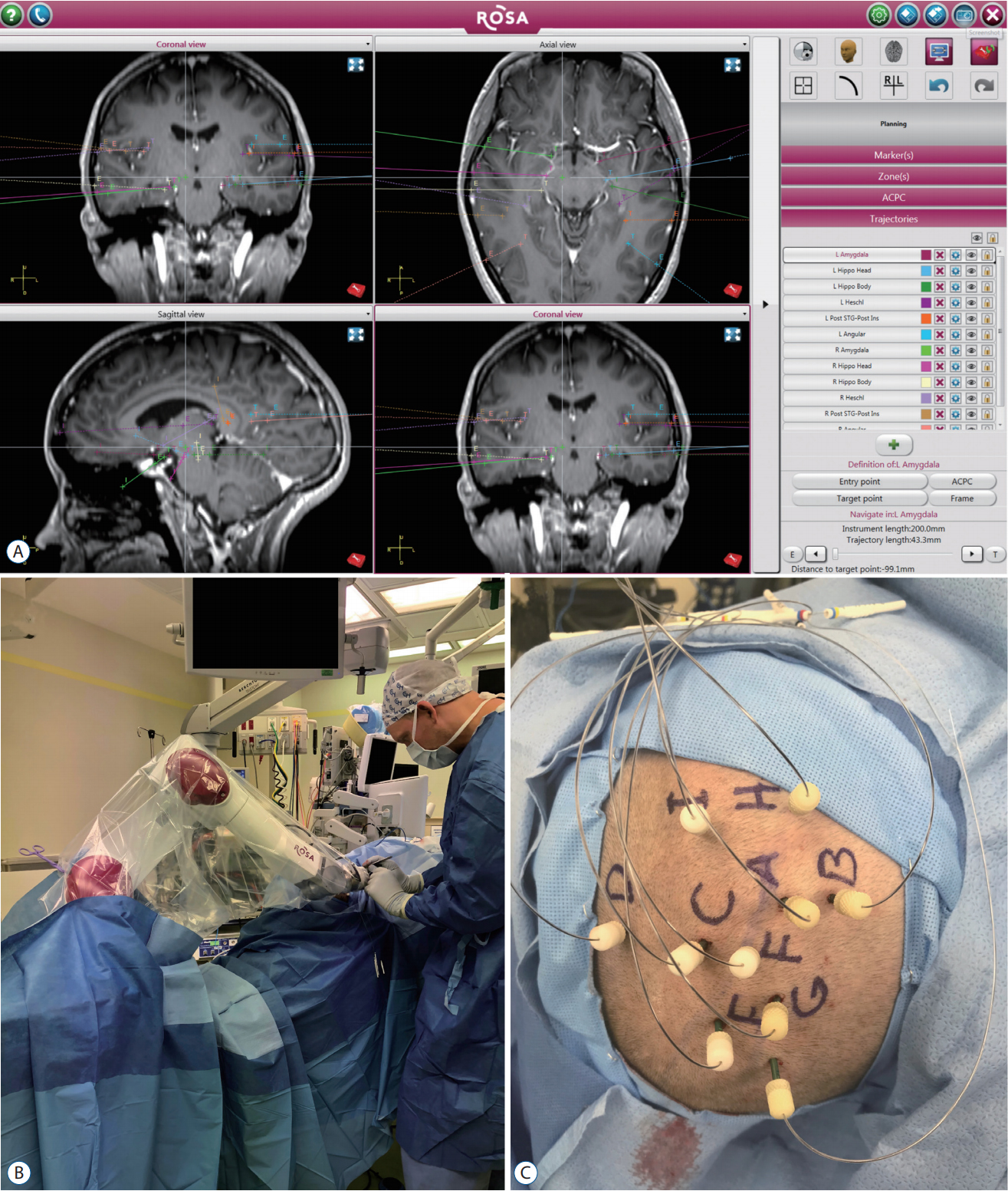
Stereoelectroencephalography (SEEG) procedure. A : The Robotic Stereotactic Assistant planning software (Zimmer Biomet, Warsaw, IN, USA), demonstrating a bitemporal SEEG plan in a patient for whom bitemporal subdural strips failed to adequately localize seizure onset. B : The robot arm moves into position for each electrode, and instruments are placed through an instrument holder on the robot arm to each stereotactic target. C : Intraoperative photograph of secured electrodes after implantation.
A unique label is assigned to each electrode (e.g., ‘Electrode A’), which is carefully documented for later reference. At some institutions, electrodes may be temporarily plugged in to confirm that recording quality is appropriate before exiting the operating room. Post-operative imaging is performed using CT and/or MRI for localization of electrode positions and to confirm the absence of procedure-related complications.
Monitoring
The interpretation of SEEG recordings shares many core principles with the reading of subdural grid recordings. In both modalities, electrographic findings of interest mainly include seizures (onset and propagation) and interictal paroxysms (e.g., spikes, sharps, high-frequency oscillations, etc.), and the duration of monitoring depends on the time needed to capture sufficient electrographic evidence to support or refute the pre-implantation hypothesis. Issues specific to SEEG interpretation include the need to consider complex three-dimensional relationships between contacts and electrodes [4], the novelty of recording from white matter [10], and the particular artifact patterns present in SEEG recordings [57]. Unlike grids, each SEEG electrode typically samples the neocortex, white matter, and deep structures (e.g., amygdala, hippocampus, insula, cingulate) via contacts at varying depths. On bipolar projections, white matter recordings appear relatively isoelectric, which can serve as a landmark to distinguish between recordings of the neocortex and deeper structures. Common artifacts present in SEEG recordings include muscle artifact in superficial contacts (especially in electrodes that pierce the temporalis muscle), 60-Hz line noise, and infrequent electrode breakage. Lastly, it bears repeating that intracranial monitoring via intracerebral electrodes constitutes only a portion of the broader SEEG methodology [17], which calls for a complete assimilation of the invasive and non-invasive findings when finalizing the treatment plan.
SAFETY, ACCURACY, AND EFFICACY
Safety
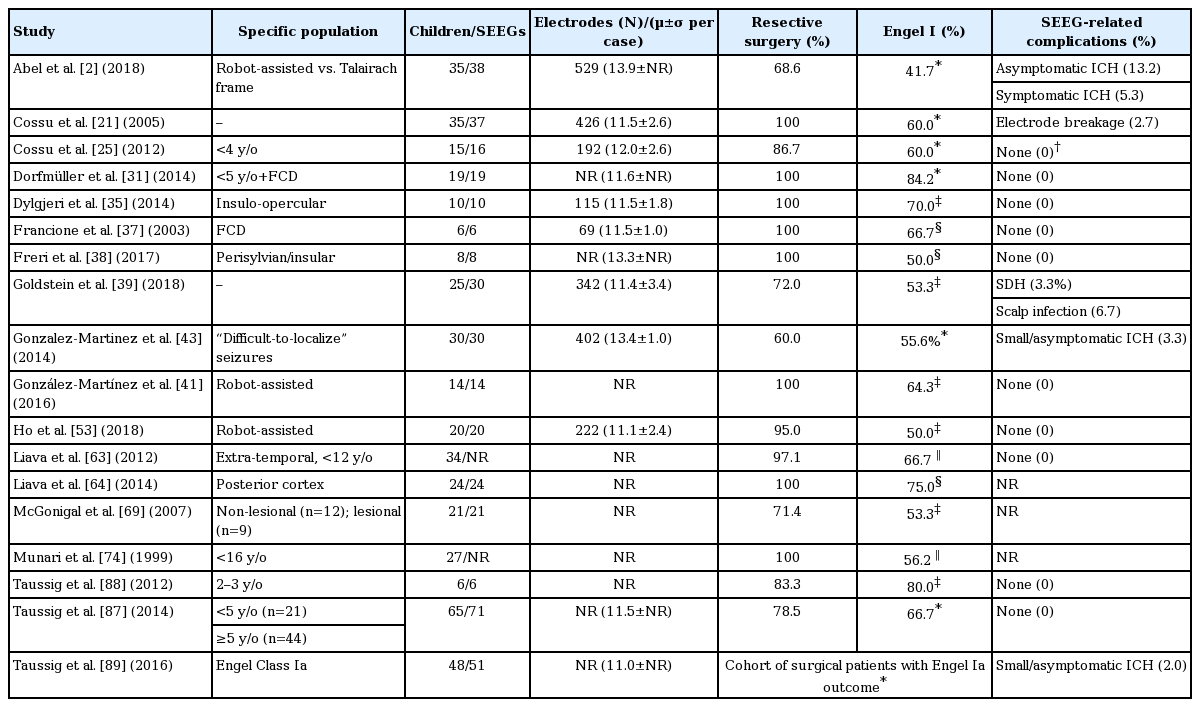
SEEG exploration is a relatively safe procedure. The majority of institutional studies examining outcomes from pediatric SEEG exploration reported either no procedure-related complications, or infrequent minor complications (Table 2). The most common SEEG-related complications are electrode breakage, superficial infection, and vascular disruption causing minor intracranial hemorrhage (ICH). Cossu et al. [21] reviewed 37 pediatric SEEG cases and identified one electrode breakage requiring surgical removal across 426 electrodes implanted (0.2% breakages/electrode). Abel et al. [2] identified four children with asymptomatic ICH, one case of transient paresthesia associated with subdural hematoma, and another with headache associated with a minor electrode-tract ICH among 38 procedures. Goldstein et al. [39] reviewed 30 SEEG explorations, finding one instance of electrode deflection resulting in asymptomatic extra-axial hemorrhage, and two cases of superficial scalp infection at the electrode entry site. These findings from pediatric series align with the more extensive literature involving adult or mixed cohorts [9,12,16,40,49,68,86], with a meta-analysis of SEEG outcomes from 2624 patients reporting a pooled prevalence of 1.0% for hemorrhagic complications and 0.8% for infectious complications [73]. Previous studies demonstrate that SEEG monitoring can be performed safely in very young children (including infants and toddlers) [25,31,87,88] though some authors suggest a minimum skull thickness of 2 mm for safe placement of anchoring bolts [55,70]. Finally, mortality associated with SEEG monitoring is extremely rare. In a meta-analysis of over 2500 SEEG explorations, mostly in adults, only five fatalities were reported (0.2%) : two attributed to ICH, two associated with complications from ventriculography, and one related to massive cerebral edema [73]. In children, only a few peri-procedural fatalities have been reported [12,25,29].
Comparison to subdural grids
Exploration with SEEG is safer and less invasive than SDG monitoring. Institutional series consistently demonstrate higher rates of hemorrhage, infection, and cerebral edema associated with SDGs [56,77,85,97]. In a large pediatric series, Blauwblomme et al. [6] reviewed 95 SDG investigations, finding that 29.8% of patients suffered a complication prolonging their hospital stay, 17.9% required unplanned reoperation, 16.8% developed subdural hemorrhage, and 14.7% developed a wound infection. Yang et al. [96] performed an institutional study comparing outcomes from SDG (n=52) and SEEG (n=48), observing higher rates of ICH and surgical site infection among SDG patients. In a meta-analysis comparing SDG and SEEG, Sacino et al. [84] reviewed 23 papers featuring 974 children (SDG, 697; SEEG, 277), finding that SDG cases had a higher pooled-prevalence of CSF leak, infection, and ICH, as well as a lower overall seizure-freedom rate (SDG, 52.1% vs. SEEG, 66.5%).
Accuracy
Accurate electrode placement is critical for sampling the desired targets and minimizing complications. Placement accuracy is typically measured in terms of entry point (EP) and target point (TP) localization errors. Comprehensive discussion of SEEG placement accuracy using frame-based, frameless, and robot-assisted techniques is available elsewhere [12,52]. A recent meta-analysis [92] found that the combined accuracy of frame-based systems (EP error, 1.43 mm; 95% confidence interval [CI], 1.35–1.51; TP error, 1.93; 95% CI, 1.05–2.81) compared favorably to frameless systems (EP error, 2.45; 95% CI, 0.39–4.51; TP error, 2.89; 95% CI, 2.34–3.44). However, localization errors <2 mm have been reported using frameless and robot-assisted techniques in both pediatric and adult populations [11,12,28,41].
Efficacy
Assessing the efficacy of SEEG is complicated. Most studies report the percentage of cases in which SEEG was “successful” or “useful” in localizing the EZ, but this definition lacks consistency across centers. The percentage of patients offered surgical resection after SEEG exploration is another flawed metric, given that SEEG findings can both rule-in or rule-out resection candidates. Additionally, post-operative seizurefreedom rates should not be confused with SEEG efficacy, as complete removal of the EZ may not be feasible even when it is well-localized (e.g., overlap with eloquent cortex).
Compared to adult patients, children with medically-refractory epilepsy (MRE) have a higher incidence of extra-temporal, non-lesional, cryptogenic, and syndromic epilepsies. SEEG may add value in these complex scenarios. In a study of extra-temporal epilepsy [63], 34/53 children (64.2%) underwent SEEG investigation, and 66.7% of SEEG patients who underwent resection were seizure-free (Engel Class I) after 12 months. McGonigal et al. [69] reviewed a large cohort of MRIlesional and non-lesional patients undergoing SEEG exploration. In the full cohort (80 adults, 20 children), “successful” EZ localization was achieved in 96% of cases (55/57 lesional, 41/43 non-lesional). Among 15 children who underwent resection, 4/8 children with non-lesional epilepsy and 4/7 children with lesional epilepsy were seizure-free after ≥6 months. Gonzalez-Martinez et al. [43] reported 30 children with “difficult-to-localize” MRE, including 66% with non-lesional, equivocal, or bilateral MRI findings. SEEG localized the EZ in 26/30 cases (86.7%), and 55.6% of children offered resection were seizure-free after 12 months. SEEG has also shown utility in specific MRE populations, including children with insular epilepsy [35], periventricular nodular heterotopia [90], and polymicrogyria [67].
THERAPEUTIC APPLICATIONS
In addition to diagnostic exploration, SEEG electrodes can be used to perform stereotactic ablation as a form of minimally-invasive epilepsy surgery. SEEG-guided radiofrequency thermocoagulation (RF-TC) is a technique used to produce focal areas of thermal injury and coagulative necrosis by delivering current through neighboring electrodes. RF-TC can be performed using the same electrodes implanted for passive recording, affording access to many lesion targets without the risk of additional electrode or laser fiber passages. Early work by Guénot and colleagues [48] established the safety and feasibility of RF-TC in a series of 20 patients with MRE, and in the years since, RF-TC has shown potential as a palliative option for a limited range of epilepsy-related indications [7,8,13,15,19,22,24,71,90,93,98]. At present, the literature reporting outcomes of RF-TC in children [14,23,30,47] suggests a marginal role of RF-TC in pediatric MRE management, and is potentially useful as palliative therapy when the patient is not a candidate for resective surgery.
Laser ablation with MR-guided laser interstitial thermal therapy (MR-g-LITT) is another minimally-invasive technique in which a fiber-optic laser applicator is stereotactically inserted, and ablation is performed while acquiring real-time thermal MRI sequences [27,45,61,62]. At some centers, SEEG electrodes are used to identify ablation targets [82]. Favorable outcomes from SEEG followed by MR-g-LITT for children with MRE have been described [18], although one study reported a relatively high rate of transient functional deficits (7/24 procedures, 29.2%) [78]. Similar to RF-TC, the precise role of MR-g-LITT in pediatric MRE treatment remains undefined.
Finally, SEEG may be used to determine the optimal targets for the electrodes of responsive neurostimulation systems [66], which are designed to arrest seizures through closed-loop stimulation [51]. This is typically employed as a treatment strategy when seizure onset arises from eloquent areas.
CONCLUSION
Epilepsy surgery is an important treatment option for children with MRE. Invasive monitoring with subdural grids or SEEG electrodes is indicated when the non-invasive evaluation fails to conclusively localize the EZ. Extensive outcomes research involving pediatric and adult patients shows that SEEG exploration is very safe. As a diagnostic method, SEEG is particularly valuable when the anatomo-electro-clinical hypothesis predicts the involvement of deep structures, bilateral networks, or after unsuccessful investigation with subdural electrodes. Therapeutically, SEEG may be used to guide ablative therapies via RF-TC or MR-g-LITT. Continued adoption by epilepsy centers worldwide promises future innovation and an expanded role of SEEG in the surgical management of pediatric epilepsy.
Notes
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.