Radiosurgical Techniques and Clinical Outcomes of Gamma Knife Radiosurgery for Brainstem Arteriovenous Malformations
Article information
Abstract
Objective
Brainstem arteriovenous malformation (AVM) is rare and radiosurgical management is complicated by the sensitivity of the adjacent neurological structures. Complete obliteration of the nidus is not always possible. We describe over 20 years of radiosurgical procedures for brainstem AVMs, focusing on clinical outcomes and radiosurgical techniques.
Methods
Between 1992 and 2011, the authors performed gamma knife radiosurgery (GKRS) in 464 cerebral AVMs. Twenty-nine of the 464 patients (6.3%) reviewed had brainstem AVMs. This series included sixteen males and thirteen females with a mean age of 30.7 years (range : 5-71 years). The symptoms that led to diagnoses were as follows : an altered mentality (5 patients, 17.3%), motor weakness (10 patients, 34.5%), cranial nerve symptoms (3 patients, 10.3%), headache (6 patients, 20.7%), dizziness (3 patients, 10.3%), and seizures (2 patients, 6.9%). Two patients had undergone a previous nidus resection, and three patients had undergone a previous embolization. Twenty-four patients underwent only GKRS. With respect to the nidus type and blood flow, the ratio of compact type to diffuse type and high flow to low flow were 17 : 12 and 16 : 13, respectively. In this series, 24 patients (82.8%) had a prior hemorrhage. The mean target volume was 1.7 cm3 (range 0.1-11.3 cm3). The mean maximal and marginal radiation doses were 38.5 Gy (range 28.6-43.6 Gy) and 23.4 Gy (range 18-27 Gy), and the mean isodose profile was 61.3% (range 50-70%).
Results
Twenty-four patients had brainstem AVMs and were followed for more than 3 years. Obliteration of the AVMs was eventually documented in 17 patients (70.8%) over a mean follow-up period of 77.5 months (range 36-216 months). With respect to nidus type and blood flow, the obliteration rate of compact types (75%) was higher than that of diffuse types (66.7%), and the obliteration rate of low flow AVMs (76.9%) was higher than that of high flow AVMs (63.6%) (p<0.05). Two patients (6.9%) with three hemorrhagic events suffered a hemorrhage during the follow-up period. The annual bleeding rate of AVM after GKRS was 1.95% per year. No adverse radiation effects or delayed cystic formations were found.
Conclusion
GKRS has an important clinical role in treatment of brainstem AVMs, which carry excessive surgical risks. Angiographic features and radiosurgical techniques using a lower maximal dose with higher isodose profiles are important for lesion obliteration and the avoidance of complications.
INTRODUCTION
Gamma knife radiosurgery (GKRS) has been widely used for the treatment of cerebral arteriovenous malformations (AVMs). Several studies have described successful radiosurgical results for AVMs located in critical areas of the brain16-18,24,27,30), but there is little data regarding long term results25,29) even though GKRS is considered a valid treatment modality14,28). GKRS is also known to be associated with serious neurological deterioration when used for brain stem lesions6,8-10), and at present, no widely accepted standard radiosurgery protocols for this area have been established. In this study, we describe the outcomes of patients with AVMs involving brainstem who underwent GKRS at a single institute over a 20-year period. The main purpose of this study was to review our experience with GKRS in the treatment of AVMs involving brainstem and to analyze the specific factors related to clinical outcomes.
MATERIALS AND METHODS
Patient selection criteria
The authors performed GKRS in 464 cerebral AVMs between 1992 and 2011. Twenty-nine of 464 patients (6.3%) were diagnosed with AVMs in the brainstem (sixteen males and thirteen females). Outcome data was obtained from a review of patient medical records. Patient ages ranged from 5 to 71 years (mean : 30.7). The initial symptoms leading to diagnosis included : an altered mentality (5 patients, 17.3%), motor weakness (10 patients, 34.5%), cranial nerve symptoms (3 patients, 10.3%), headache (6 patients, 20.7%), dizziness (3 patients, 10.3%), and seizures (2 patients, 6.9%). Before GKRS, 24 patients (82.8%) had a history of hemorrhage from AVMs. Nine patients had undergone prior surgical intervention, including hematoma evacuation (2 patients), nidus resection with hematoma evacuation (2 patients), ventricular drainage (2 patients), ventricular drainage with embolization (1 patients), and embolization (2 patients). Twenty patients underwent GKRS alone (Table 1).
Radiologic features of AVM
In all cases, the AVM characteristics were determined using cerebral angiography and MRI. The AVM locations were the thalamus+midbrain region in 14 patients, the midbrain region in 9 patients, the pons region in 4 patients, and the medulla oblongata in 2 patients (Table 1). Pial or subpial lesions were excluded from this study. The Spetzler-Martin grade was Grade III in 28, Grade IV in 1, and Grade V in 0. The AVMs were supplied mainly by a posterior cerebral artery (3), thalamoperforator (11), posterior choroidal artery (7), superior cerebellar artery (3), anterior inferior cerebellar artery (3), and posterior inferior cerebellar artery (2), and 11 had multiple feeders. AVMs drained into the galenic system in 23 cases and the petrosal vein in 6 cases. Nidus type were dichotomized into two groups according to enhanced vessel volume in nidus/total nidus volume ratio (V/N ratio) : compact type (0.7< V/N ratio) and diffuse type (0.7≥ V/N ratio)17,26). Compact types were found in 17 AVMs and diffuse types in 12. Hemodynamically, AVMs were dichotomized into two groups : high flow AVMs (draining vein was showed before early capillary phase in angiography), and low flow AVMs (draining vein was showed after capillary phase in angiography)17,26). High flow AVMs were found in 16 cases and low flow AVMs were found in 13 cases (Table 2).
Radiosurgical techniques
All patients underwent application of an imaging-compatible stereotactic frame (Elekta Instruments AB, Stockholm, Sweden) after the administration of local anesthetics. Stereotactic magnetic resonance (MR) imaging studies, including a T1-weighted spin echo, T2-weighted, T1-weighted enhanced, and time of flight imaging sequences with biplane stereotactic angiography were obtained to demonstrate and target the lesions. Dose planning was performed using the KULA planning system (Elekta Instruments AB, Stockholm, Sweden) until December 2001, and using the Leksell Gamma Plan (Elekta Instruments AB, Stockholm, Sweden) after January 2002. Multiple isocenter dose planning with a higher isodose profile was performed to create conformal dose plans with lower maximal doses and larger hot spots, and to minimize irradiation of adjacent critical brain tissue (Fig. 1). GKRS was performed using a Model B or Perfexion Leksell Gamma Knife (Elekta Instruments AB, Stockholm, Sweden). Patients were discharged the day following GKRS.

Radiosurgical dose planning for a pontine AVM. A : Dose planning using a 50% isodose profile. B : Dose planning using a 60% isodose profile. The hot spot (80% isodose profile) in Fig. 1B is larger than in Fig. 1A. On the other hand, the 8 Gy and 12 Gy volumes of Fig. 1B are smaller than those of Fig. 1A. AVM : arteriovenous malformation.
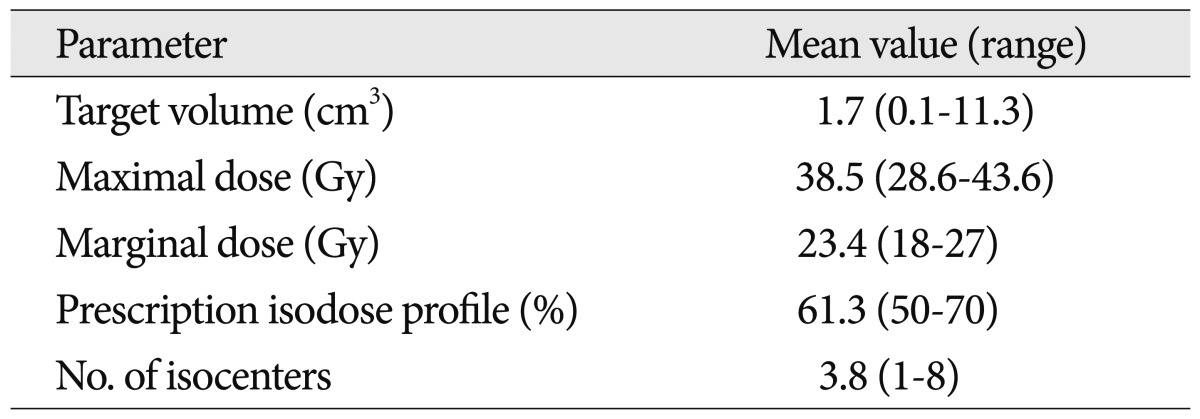
The mean target volume at the time of GKRS was 1.7 cm3 (range 0.1-11.3 cm3). The mean prescription isodose profile was 61.3% (range 50-70%), the mean dose delivered to the margin of the malformations was 23.4 Gy (range 18-27 Gy), and the mean maximum dose was 38.5 Gy (range 28.6-43.6 Gy). Patients were treated using multiple isocenters (mean 3.8; range 1-8) (Table 3).
Follow-up evaluation
Follow-up MR imaging was performed at 12-month intervals. If MR imaging suggested complete obliteration at the end of 3 years, then a repeat angiogram was requested. If the MR image showed a clearly defined a residual nidus, follow-up angiography and additional treatment were delayed. When obliteration of the AVM was suggested by MR imaging, cerebral angiography was requested to confirm the obliteration. Complete obliteration was defined as disappearance of the AVM nidus and venous shunt. If new or worsening clinical symptoms, hemorrhaging, or adverse radiation effects (AREs) developed, they were ruled out using additional imaging studies.
All images were reviewed by two neurosurgeons and one neuroradiologist. A comparison of lesion volume at the time of treatment was compared to lesion volumes in previous images. The images were also assessed for radiation-induced changes to the brain.
RESULTS
Clinical outcomes
At initial assessment, all patients were symptomatic. Five patients showed altered mentality (17.3%), ten showed motor weakness (34.5%), three showed cranial nerve symptoms (10.3%), six had headaches (20.7%), three showed dizziness (10.3%), and two had symptoms of seizures (6.9%). At the last follow-up (between 12 months to 208 months), twelve patients remained symptom-free. Eight patients improved significantly but still had focal neurologic deficits. Nine patients had fixed neurologic deficits which had existed before GKRS.
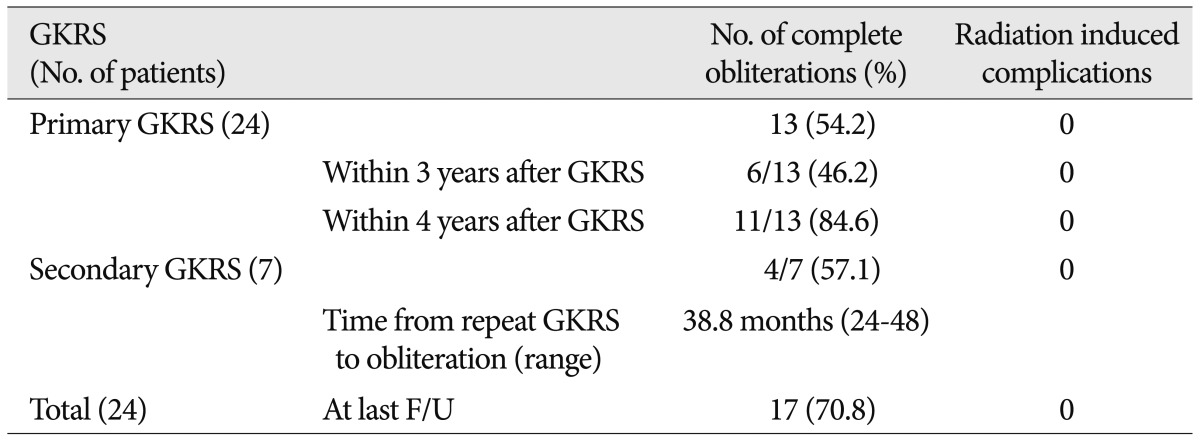
Obliteration rate after a single radiosurgical session (Table 4)
The mean clinical follow-up time for the twenty-nine patients was 65.1 months (range 12-216 months). Among them, twenty-four were followed for more than 3 years. Complete obliteration of the AVMs was eventually documented in 17 patients (70.8%) at a mean follow-up of 56.0 months (range 24-208 months), and complete obliteration of the AVMs with a single GKRS was eventually documented in 13 patients (54.2%) at a mean follow-up of 37.2 months (range 24-72 months). Among these patients, single GKRS achieved complete obliteration in 6 of 13 (46.2%) at 3 years after GKRS, and in 11 of 13 (84.6%) within 4 years of GKRS. An additional GKRS was performed in seven patients. Seven patients underwent repeat GKRS and one patient underwent a third GKRS procedure (Fig. 2). The mean target volume at the time of repeat GKRS was 1.3 cm3 (range 0.1-4.6 cm3). The mean prescription isodose profile was 62.2% (range 50-70%), the mean marginal dose was 21.7 Gy (range 17.5-25.2 Gy), and the mean maximum dose was 35.1 Gy (range 25-40 Gy). The mean number of isocenters was 3.6 (range 1-9). In the repeat GKRS group, 4 patients (57.1%) had complete obliteration of the nidus based on angiography. Complete obliteration occurred 24-48 months after the last radiosurgery (mean 38.8 months) (Table 5).
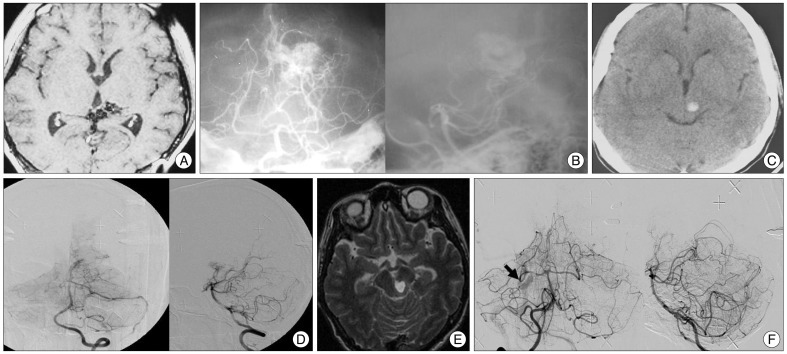
A : A T1 weighted contrast enhanced magnetic resonance (MR) image of a 21-year-old female before her 1st radiosurgery showing midbrain bleeding. B : A stereotactic vertebral artery angiography showing a nidus. C : A CT image 10 years after the first radiosurgery showing midbrain rebleeding. D : A digital subtraction angiography (DSA) for the second radiosurgery showing a remaining nidus (missed target). E : A T2 weighted MR image 15 years after the 1st radiosurgery showing rebleeding. F : A DSA for the 3rd radiosurgery showing remaining arteriovenous shunting (black arrow).
Factors related to obliteration
By univariate analysis, a target volume of <2 cc, compact nidus type, AVM low flow type, and low flow with compact nidus were significantly associated with a higher rate of total obliteration on angiography (p<0.05) (Table 6). With respect to nidus type and blood flow, the obliteration rate for compact types (75%) was higher than for diffuse types (66.7%), and the obliteration rate in low flow AVMs (76.9%) was higher than in high flow AVMs (63.6%) (p<0.05).
Hemorrhages and complications after GKRS
Two patients (6.9%) experienced three hemorrhagic events. The intervals between GKRS and the hemorrhagic events ranged from 84 to 180 months (84, 108, and 180 months). In one patient, the AVM was located in the midbrain, while in another, it was in the pons. In the first patient, a hemiparesis developed, but resolved without treatment. The other patient suffered mental deterioration that resolved without surgical interventions. The annual bleeding rate after GKRS was 1.95% per year.
No adverse radiation effects (AREs) or delayed cyst formations occurred.
DISCUSSION
General aspects and treatment modalities of brainstem AVMs
AVMs of the brainstem represent 2-6% of all cerebral AVMs7,15,17). Several studies have stated that brain AVMs have a high risk of bleeding. About 80-90% of patients initially present with an intracranial hemorrhage7,25), and history of an untreated vascular malformation could lead to a high risk of morbidity or mortality2,3,5,11,22,23). The cumulative risk of hemorrhage along with the associated high morbidity and mortality in patients with AVMs emphasizes the need for treatment in patients with brainstem AVMs. However, the best options for management of brainstem AVMs remain controversial. Surgical approaches for carefully selected patients have been reported for these difficult vascular malformations, but most of these reports are based on a small series involving highly selected cases or case reports4,7,12,16,22,25). These reports indicate that although pial or subpial lesions can be safely extirpated by surgery, attempts to resect lesions with an intra-axial component lead to high complication rates and a low rate of complete nidus removal.
Endovascular treatment is rarely curative and is typically performed as a preoperative adjuvant therapy in limited cases before microsurgery or GKRS, because embolization within the brainstem is potentially very dangerous and the feeder of the AVMs often also supplies the surrounding brainstem16,25).
Radiosurgery for brain stem AVMs
GKRS has been widely used for the treatment of brain AVMs. Several studies have described successful outcomes for AVMs located in critical areas of the brain16,18,27,30), but there is little data on the long-term results of brainstem AVMs, as it is a relatively new treatment modality for these types of AVMs25). Although GKRS is considered to be a valid treatment14,28), it is also known to cause serious neurological deterioration when used for brainstem lesions1,6,8,10).
Prior studies have indicated that total obliteration rates of brainstem lesions after a single procedure vary between 52% and 73% (Table 7)15,19,20). These rates are lower than the total obliteration rates for AVMs in other locations, which have been reported to be between 70-87%19). In this study, the complete obliteration rate after single GKRS was 54.2%. This rate is relatively similar to those of other studies. However, this result is lower than rates for other AVM locations because of the dose reduction necessary for avoiding AREs.
Complications of GKRS
Many studies have noted that the risk of AREs in patients with brainstem AVMs is higher than in patients with AVMs in other locations13,20). Following GKRS, radiation-induced complication rates have been reported as being approximately 11.8%. However, in the present series, radiation-related complications did not arise (Table 6). Kano et al.13) reported that factors associated with a higher rates of symptomatic AREs included a larger 12 Gy volume and a higher Spetzler-Martin grade. By using higher isodose profiles, patients received not only adequate marginal doses but also lower maximal doses with a larger hot spot area (>80% of maximal doses) which can induce similar outcomes with lower complication rates (Fig. 1). Further studies are necessary to confirm the advantages of this technique.
Factors associated with complete obliteration
Factors related to complete obliteration following GKRS have been reported to be tumor marginal dose, the number of isocenters, nidus volume, sex, nidus location, and the number of draining veins15,19). In this study, a volume of <2 cc, compact nidus type, and AVM low flow type were statistically significant factors associated with complete obliteration. Because most brainstem AVMs had undergone GKRS with an optimal marginal dose of >20 Gy, a marginal dose of >20 Gy was not significant statistically. However the authors believe that a marginal dose of >20 Gy is an important factor associated with complete obliteration.
Hemorrhage after GKRS
AVMs in infratentorial locations are twice as likely to present with a hemorrhage as supratentorial AVMs21,26). Between 70% and 90% of patients with brainstem AVMs present with a bleeding event15,19,25). In this study, twenty-four AVMs (82.8%) presented with a hemorrhagic event. Many studies have reported that the annual risk of hemorrhage after GKRS is 1.9-4.0%13,15), and our result of 1.95% is similar to this, though slightly lower. Kano et al.13) suggested that lower annual bleeding rates are related to higher margin doses. In a report by Kurita et al.15), patients received marginal doses similar to the doses (18-20 Gy) and suffered higher annual bleeding rates. We note that almost all patients in this study received a standard dose (>20 Gy).
CONCLUSION
Although GKRS for brainstem AVMs has a relatively lower obliteration rate and higher complication rate than for AVMs in other locations, GKRS is an effective treatment modality for brainstem AVMs, which carry excessive surgicial risks. A pre-GKRS angiographic finding [compact nidus, low flow, and small target volume (<2 cc)] and optimal marginal dose with higher isodose profile were associated with avoiding post-procedural AREs and achieving complete obliteration.