The Clinical Efficacy of Decompressive Craniectomy in Patients with an Internal Carotid Artery Territory Infarction
Article information
Abstract
Objective
To evaluate the surgical efficacy of and factors associated with decompressive craniectomy in patients with an internal carotid artery (ICA) territory infarction.
Methods
Seventeen patients (8 men and 9 women, average age 61.53 years, range 53-77 years) were treated by decompressive craniectomy for an ICA territory infarction at our institute. We retrospectively reviewed medical records, radiological findings, and National Institutes of Health Stroke Scale (NIHSS) at presentation and before surgery. Clinical outcomes were assessed using the Glasgow Outcome Scale (GOS).
Results
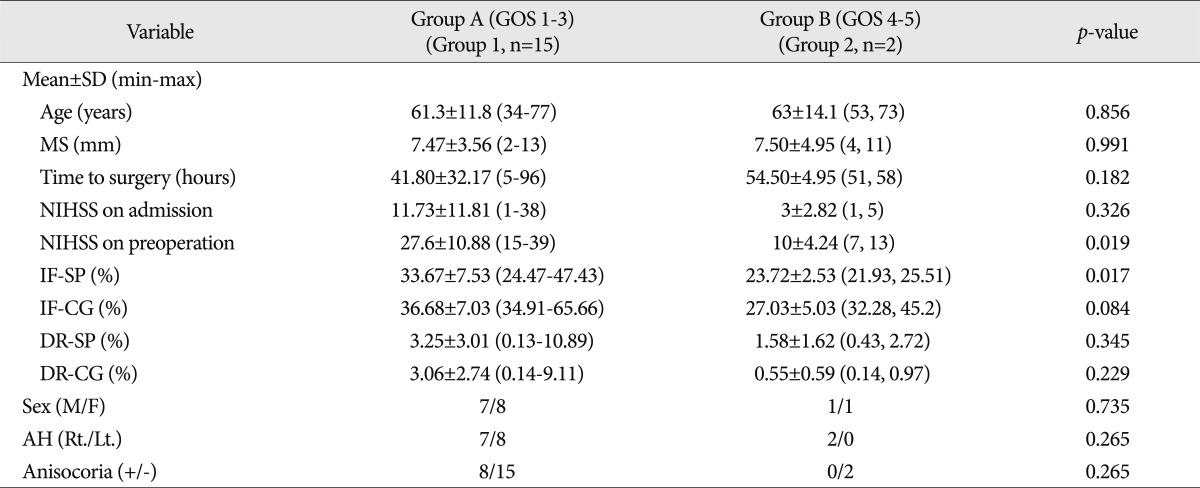
Of the 17 patients, 15 (88.24%) achieved a poor outcome (Group A, GOS 1-3) and 2 (11.76%) a good outcome (Group B, GOS 4-5). The mortality rate at one month after surgery was 52.9%. Average preoperative NIHSS was 27.6±10.88% in group A and 10±4.24% in group B. Mean cerebral infarction fraction at the septum pellucidum level before surgery in group A and B were 33.67% and 23.72%, respectively. Mean preoperative NIHSS (p=0.019) and cerebral infarction fraction at the septum pellucidum level (p=0.017) were found to be significantly associated with a better outcome. However, no preexisting prognostic factor was found to be of statistical significance.
Conclusion
The rate of mortality after ICA territory infarction treatment is relatively high, despite positive evidence for surgical decompression, and most survivors experience severe disabilities. Our findings caution that careful consideration of prognostic factors is required when considering surgical treatment.
INTRODUCTION
Malignant cerebral infarction is a large hemispheric infarction caused by a middle cerebral artery (MCA) or internal carotid artery (ICA) occlusion. This state occurs in between 10 and 15% of all cerebral infarction patients, and has a mortality rate of up to 80%, despite maximum conservative care7,17,32). Decompressive craniectomy and duraplasty are widely performed to reduce intracranial pressure and improve circulation to reduce the risk of mortality in cerebral infarction patients3,6,32), and several authors have reported that surgical decompression lowers the mortality rate to between 16 and 42 percent4,5,11,13,17,20), but in most of these studies, infarctions were limited to the MCA territory. On the other hand, Klincer et al. reported surgical results for ICA territory infarctions in 10 of 25 malignant infarction patients that underwent surgical decompression, and concluded that surgical treatment provided no benefit18).
Since involvement by an ICA territory infarction is wider than that of a MCA territory infarction and cerebral edema is more severe, it is difficult to predict the efficacy of decompressive surgery. In the present study, we studied the usefulness of surgical decompression in patients with an ICA territory infarction and sought to identify prognostic factors.
MATERIALS AND METHODS
Patient selection
This retrospective study was performed on 17 patients that underwent decompressive surgery due to a malignant cerebral infarction from January 1992 to December 2010. Patients all had a diagnosis of an ICA territory infarction by computed tomography (CT), CT angiography or magnetic resonance angiography. Furthermore, all exhibited neurologic condition or brain herniation aggravation by imaging studies despite the best possible medical treatment available.
Initial treatment
Our policy dictates that all patients with an ischemic stroke condition should be admitted to an acute stroke unit under the care of the Neurology Department. We administer tissue plasminogen activator to patients that arrive within three hours of symptom development and anticoagulants, such as, aspirin (100 mg) and clopidogrel (75 mg), to patients that arrive later. In the event of neurological deterioration and the presence of radiologic evidence of impending hernia secondary to cerebral infarction, maximum medical treatment including osmotherapy, steroid administration, hyperventilation, hypothermia treatment and neurosurgical consultation is instituted.
In these settings, unless a patient has an overwhelming medical contraindication that prohibits surgery, decompressive craniectomy and duraplasty are performed. No antidote for anticoagulants is administered prior to surgery.
Data collection
The following data were collected; patient age, gender, the cerebral hemisphere affected, anisocoria, National Institutes of Health Stroke Scale (NIHSS) on admission and before surgery, degree of midline shifting on CT scans, cerebral infarction fractions at the septum pellucidum and cingulate gyrus levels by CT before surgery, time from hospitalization to surgery, and the decompression ratios (defined below) at the same levels in same CT scan. Surgical outcomes were evaluated using the Glasgow Outcome Scale (GOS) one month after surgery.
Dimensional study
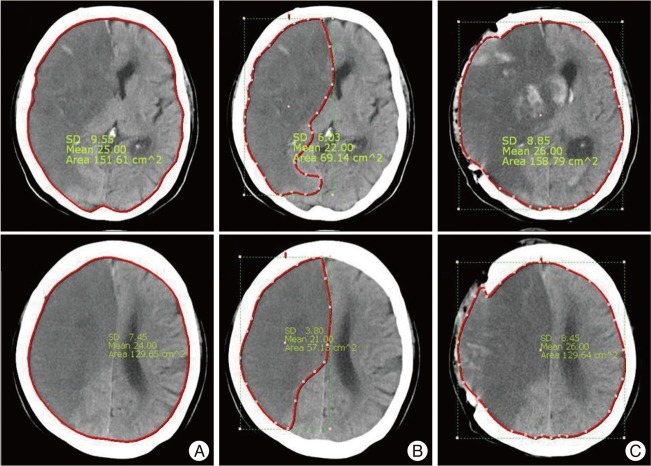
We measured total brain areas as well as infarction areas using preoperative brain CT scans taken at the septum pellucidum and cingulate gyrus levels. In order to evaluate infarction severities, we defined infarction fraction as the percentage of total brain area occupied by a low density lesion at each level (Fig. 1).
In addition, we defined decompression ratio as the ratio of postoperative extended area to preoperative total brain area at the septum pellucidum and cingulated gyrus levels on CT scans, in the belief that this value would provide a means of indirectly evaluating the effectiveness of decompression [Fig. 1 (C-A)/A]. The Maroview picture archiving and communication system (PACS; Infinitt) was used to measure areas in the brain CT images.
Surgical technique
The principal surgical technique was decompressive craniectomy with duraplasty. Surgery was performed with the patient lying supine under general anesthesia. Using a modified large Dandy skin incision, frontotemporoparietal craniectomy was performed through a muscular approach. A larger bone flap (minimal diameter >12 cm) than is usual for MCA infarction was made and the temporal squama was removed with a rongeur to prevent uncal herniation until the mid cranial fossa was exposed. A stellate incision was then made on the dura mater to allow the brain to expand outward and artificial dura (Lyodura®, B.Braun) was used as an on-lay graft. Postoperatively, all patients were sent to the neurological intensive care unit for close monitoring and management.
Outcome analysis
GOS was used to measure clinical outcomes at one month after surgery. Patients were divided into two groups according to GOS grades. Patients of GOS 1-3 were allocated to group A, and those of GOS 4-5 to group B.
Statistical analysis
All results are expressed as means and standard deviations. SPSS 19.0 was used for the analysis. Statistical significance was accepted for p value <0.05.
RESULTS
All 17 patients underwent decompressive craniectomy and duraplasty. Eight patients were men and nine were women. Average patient age was 61.53±11.64 years (range from 53 to 77 years). NIHSS on admission at the department of neurology was 10.71±11.44 (range from 1 to 38) whereas NIHSS immediately before surgery, was 25.53±11.79 (range from 7 to 39). Eight patients (47.05%) showed anisocoria and all patients appeared to have hemiplegia. Eight patients (47.05%) had infarctions in the dominant hemisphere, and nine patients in the non-dominant hemisphere (52.94%).
The mean degree of midline shift in CT scans taken before surgery was 7.47±3.55 mm (range from 2 to 13 mm). Mean time from symptom development to surgery was 43.29±30.41 hours (range from 5 to 96 hours). No surgically related complication occurred. Two patients (11.8%) were moderately disabled within one month after surgery and six (35.2%) presented in a severely disabled state. Nine patients (52.9%) died within two weeks of surgery due to cerebral edema progression (Table 1).
Age and sex
Group A was composed of seven men and eight women of average age 61.33±11.8 years (range from 34 to 77 years), whereas group B was composed of one man and one woman of average age 63 years. Between two groups, there was no statistically significant difference in age and sex.
NIHSS
In group A, mean NIHSS scores on admission and before surgery were 11.73±11.81 (range from 1 to 38) and 27.6±10.88 (range from 15 to 39), respectively, and in group B were 3±2.83 (1, 5) and 10±4.24 (7, 13), respectively. The average NIHSS on admission and before surgery were lower in group B. Preoperative NIHSS scores were significantly different in the two groups (p=0.019).
Midline shift and timing of surgery
In group A, mean degree of midline shift in imaging studies before surgery was 7.47±3.56 mm (range from 2 to 13), and in group B was 7.5±4.95 mm (4, 11). In group A, mean time taken from symptom development to surgery was 41.8±32.17 hours (range from 5 to 96 hours), and in group B was 54.5±4.95 hours (51, 58), which did not represent a significant difference.
Affected hemisphere and presence of anisocoria
In group A, eight patients had a cerebral infarction in the dominant hemisphere and seven in the non-dominant hemisphere. The two patients in group B had infarctions in the non-dominant hemisphere, but there was no significant difference between the two groups (p=0.265). Eight patients in group A had anisocoria, whereas no patient in group B had anisocoria. However, no significant intergroup difference was evident.
Cerebral infarction fraction
In group A, mean infarction fraction before surgery at septum pellucidum level was 33.67%, whereas in group B, this was 23.72%, which appeared to be significantly different (p=0.017). Mean infarction fractions at the cingulate gyrus level in groups A and B were 36.68% and 27.03%, respectively.
Dimensional increases in area
On brain CT scan taken at the septum pellucidum and cingulate gyrus levels, decompression ratios were 3.25%, and 3.06%, respectively, in group A and 1.58%, and 0.55% in group B. No significant intergroup difference was found (Table 2).
DISCUSSION
Large hemispheric infarctions due to MCA or ICA occlusion constitute a major cause of severe morbidity and mortality40). Cerebral edema develops as an immediate consequence of cerebral infarction and its severity is closely related to degree of cerebral infarction24). Severe, life-threatening brain edema occurs in about 10% of patients with a large hemispheric infarction, and the majority of these patients succumb to an increase in intracranial pressure and subsequent uncal herniation26).
Hacke et al.9) defined 'malignant cerebral infarction' as a subtype of stroke, that presents itself clinically with severe hemispheric stroke syndrome and almost always indicates death due to herniation, despite maximum medical treatment for identified brain edema. When this clinical presentation is accompanied by early CT signs of major infarct during the first 12 hours after stroke and a distal internal carotid artery or proximal MCA plus anterior cerebral artery occlusion, massive hemispheric swelling occurs during the subsequent 24-72 hours8). Furthermore, most of these patients rapidly deteriorate within 2-4 days18,32,35). In cases of malignant cerebral infarction, mortality is exceptionally high and severe neurological deficits may develop despite conservative treatment, including intensive care, head elevation, sedation, hyperventilation, osmotherapy, and hypothermia treatment, in an intensive care unit2,6,9).
Decompressive craniectomy was first reported in the 1950's as a more aggressive treatment for malignant cerebral infarction19). The number of positive outcomes after decompressive craniectomy in malignant cerebral infarction increased since brain CT became widely available in the 1980's3,6,8,17,30,32). Forsting et al.8) reported that the decompressive craniectomy per se increases leptomeningeal collateral and cerebral perfusion pressures, and not only lowers mortality but also reduces cerebral infarction size and improves prognosis. Furthermore, Bendszus et al.1) in a perfusion CT study, reported that ischemic changes in areas that were initially unaffected by infarction were reduced after decompressive craniectomy.
Surgical decompression plays a certain role in treating malignant cerebral infarction, as it lowers the mortality rate and improves functional outcomes, but most studies on the subject addressed MCA territory infarction. Acute ICA infarction is recognized to be a rare, but critical disease, the outcomes of patients with a cerebral infarction due to an acutely occluded ICA are poor. In fact, only 2-12% achieve a good outcome, 16-55% succumb to a complication, and 40-69% are severely disabled23). Trouillas et al.34) in a multivariate regression analysis of 100 patients, concluded that proximal ICA occlusions are associated with a poor outcome. Although the natural history of patients with a cerebral infarction due to an acutely occluded ICA is known to be dismal, its clinical outcomes can take many different forms that are dependent on the collateral circulation. Powers et al.27,28) considered that the collateral circulation was critical to maintain adequate cerebral perfusion in patients with an ICA infarction, and several studies have shown that adequate collateral circulation may prevent the progression of hemodynamic failure11,15,37). For these reasons, an ICA infarction and ICA territory infarction should be regarded as different entities.
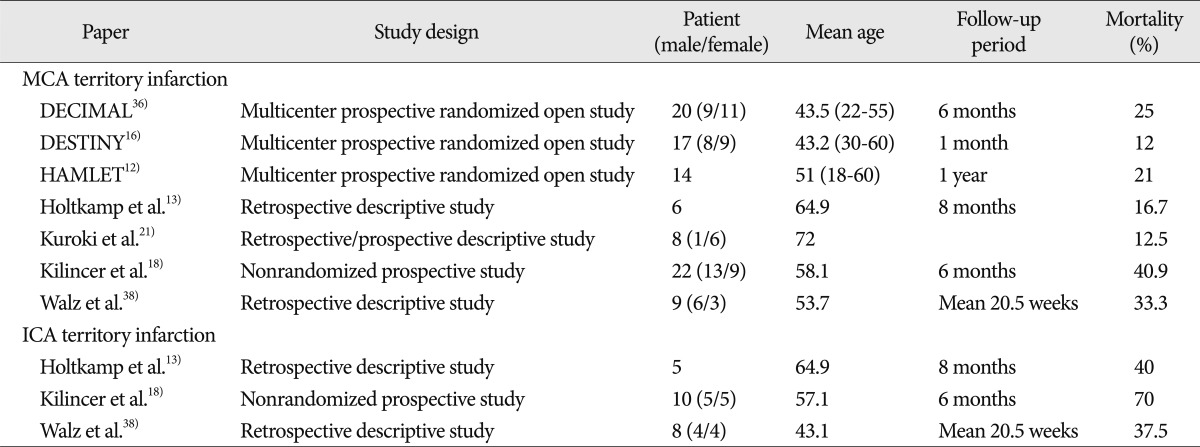
Although mortality among patients with ICA territory infarction is generally higher than among patients with an isolated MCA territory infarction (Table 3), few have reported associated factors and outcomes of decompressive surgery in patients with an ICA territory infarction. Kilincer et al.18) reported that three of 10 patients that underwent surgical decompression for an ICA territory infarction died and seven survived with severe disabilities, and concluded that surgical treatment for an ICA territory infarction was unhelpful, except in young patients, or when the infarction affected only the nondominant hemisphere. Walz et al.38) also reported that of eight ICA territory infarction patients, three died, one remained in a severely disabled state, and four remained in a moderately disabled state. Chen et al.4) found that the factors associated with higher mortality were an age of ≥60 years, involvement of more than one vascular territory, and signs of clinical herniation before surgery. The authors recommended that patients with an infarction involving more than one vascular territory be viewed as unsuitable for decompressive surgery. Our results are broadly similar to those mentioned above, as nine of our 17 patients died, three remained in a vegetative state, and three remained in a severely disabled state. Furthermore, the mortality rate was 53% and 82% achieved a poor outcome. Unfortunately, the studies by Walz et al.38) and Chen et al.4) did not identify any meaningful risk factors of poor outcomes. In the present study, we selected patients with an ICA territory infarction and evaluated prognoses using prognostic factors of malignant cerebral infarction.
Many factors that affect surgical outcome in cases of malignant cerebral infarction have been reported18,29,39). Kilincer et al.18) concluded that an advanced age (>60 years), preoperative midline shifts ≥10 mm, Glasgow Coma Scale (GCS) of ≤7, preoperative anisocoria, neurological deterioration within three days of stroke, and ICA territory infarction are factors of a poor prognosis. Park et al.25) reported that diabetes mellitus, a dominant-hemisphere infarct and a low preoperative GCS of ≤7 were poor prognostic factors for severe brain infarction.
Age could be a key prognostic factor of surgical outcome in cases of malignant cerebral infarction. Wijdicks and Diringer41) examined mortality among 42 MCA infarction patients, and found a mortality rate of 28% for patients ≤45 years and of 90.9% for those ≥45 years. In addition, Harscher et al.10) reported that the mortality rate could be lowered to 20%, if surgery was performed early in patients under 50 years of age. In the present study, age was found to have a negative effect on outcome.
Many researchers have argued that early surgery might reduce the mortality rate, because delayed surgery could cause ischemic injury in the brain stem and worsen the prognosis3,9,20,31). Carter et al.3) argued that early surgery has better outcomes than late surgery, but Harscher et al.10), Walz et al.38), Holtkamp et al.13), and Cho et al.5) reported no significant correlation between time from onset to surgery and outcomes. In the present study, the timing of surgery was not significantly different in the two groups.
Relations between surgical outcomes and preoperative neurologic conditions are also controversial. Steiger33) reported that of early neurologic findings, degree of motor paralysis and decreased mentality most importantly determine prognosis. Mattos et al.22) found that a GCS score of ≤8 before surgery was associated with poorer prognosis, but Walz et al.38) found that the surgical outcome of malignant MCA infarction was not related to initial NIHSS. In the present study, average preoperative NIHSS in group B was significantly lower than in group A (p=0.019). Thus, it seems that a lower preoperative NIHSS might be a positive prognostic factor for a good outcome.
As relations between functional outcomes and dominancy, Harscher et al.10) reported no significant difference between left and right hemispheres, whereas Wang et al.39) argued that prognosis is poorer in older patients and in patients with a dominant hemisphere infarction. In the present study, no significant difference in dominancy was found between the two groups.
Anisocoria could be a referential factor for determining the merits of surgical decompression. Cho et al.5) reported poor functional outcomes in anisocoric patients with a loss of light reflex, and thus, recommended that surgery be scheduled before light reflex is lost. In contrast, Rabinstein et al.29) found that a change in pupil size was not related to prognosis. In the present study, no significant prognostic difference was found with respect to the presence of anisocoria, but we cannot exclude the possibility that dominancy and the presence of anisocoria are not related to prognosis because our sample size was small and both patients in group B had a non-dominant infarction and no anisocoria.
Kilincer et al.18) studied the relationship between midline shifts in pre-surgical CT scans and prognosis, and reported that a midline shift of ≥10 mm is related to a poor outcome. Huh et al.14) also reported that prognosis was poorer when the midline shift was ≥11 mm. On the other hand, Rabinstein et al.29) found no relation between midline shift and functional outcomes, and similarly, in the present study, we found no significant difference between midline shifts in our two groups.
Wartenberg40) reported that the only valid predictor of a malignant course seems to be an infarction size of ≥50% of the MCA territory by CT and ≥145 mL on diffusion weighted images obtained within 14 hours of stroke onset. In present study we considered broader infarction areas, and in order to evaluate the nature of the relation between degree of cerebral infarction and surgical efficacy, we included in our analysis cerebral infarction fraction before surgery, the ratio of area increase caused by brain herniation after surgery, and midline shift. Cerebral infarction fraction in group A was 33.67±7.53% (range from 24.47 to 47.43), which was significantly smaller than in group B, 23.72±2.53% (21.93, 25.51) (p=0.017). This finding suggests that clinical results are better for smaller infarction fractions for ICA territory infarction. Decompression ratios in group A and B were 3.25±3.01% (range from 0.13 to 10.89) and 1.58±1.62% (0.43, 2.72), respectively, which were not significantly different (p=0.345). Although the decompression ratio was larger in group A, the prognosis was poor. This may be due to broader area of infarction and more severe neurological damage.
Our results show that the results of decompressive surgery for an ICA territory infarction are unsatisfactory, and that preexisting prognostic factors, such as, age, time to surgery, presence of anisocoria, and degree of midline shift are not obviously associated with outcome. Nonetheless preoperative NIHSS and infarction fraction were found to be viable prognostic factors that could be helpful when considering surgery.
However, we confess that our findings are compromised by small cohort size, and thus, we suggest that a substantially larger-scale prospective study be conducted.
CONCLUSION
We believe that decompressive craniectomy reduces mortality and improves functional outcome in cases of malignant cerebral infarction. However, the mortality rate of ICA territory infarction is high despite maximal medical treatment and surgical decompression and most survivors have severe disabilities. Therefore, when determining surgical treatment in patients with an ICA territory infarction, we recommend, in addition to prognostic factors, such as, preoperative NIHSS, and infarction fraction, that quality of life, and caregiver's burden be carefully considered.