Bae and Hyun: Endovascular Thrombectomy for Acute Ischemic Stroke : Current Concept in Management
Abstract
Endovascular thrombectomy (EVT) has been established as the standard of care in the treatment of acute ischemic stroke (AIS) based on landmark randomized controlled trials. Nevertheless, while the strict eligibility of EVT for AIS patients restrict the wide application of EVT, a considerable population still undergoes off-label EVT. Besides, it is important to acknowledge that recanalization is not achieved in approximately 20% of procedures, and more than 50% of patients who undergo EVT still do not experience a favorable outcome. This article reviews the brief history of EVT trials and recent progressions in the treatment of AIS, with focusing on the expanding eligibility criteria, new target for EVT, and the evolution of EVT techniques.
Key Words: Ischemic stroke · Thrombectomy · Mechanial thrombolysis.
INTRODUCTION
Reopening occluded vessels through endovascular thrombectomy (EVT) has improved clinical outcomes in acute ischemic stroke (AIS) by facilitating reperfusion and salvaging brain tissue at risk. The history of EVT for AIS can be described as a history of conquest and pioneers. It began with the initial exploration of intravenous thrombolysis (IVT) territory for patients with AIS caused by anterior large vessel occlusion (LVO). The subsequent territorial expansion began with widening the time window for treatment and has since continued to progress. However, despite these advancements, a vast and largely uncharted domain remains waiting to be reclaimed by further advancements in EVT.
HISTORY
IVT has been the primary treatment for AIS patients presenting within 3-4.5 hours of symptom onset since the NINDS (National Institute of Neurological Disorders and Stroke) trial in 1995 and the ECASS III (European Cooperative Acute Stroke Study) trial in 2008 [ 32, 70]. However, low recanalization rates were prominent with IVT, particularly in cases of LVO, when compared to AIS without LVO [ 11, 60]. Seven pivotal randomized controlled trials (RCTs) published between 2015 and 2016 confirmed that EVT is considered a standard treatment for patients with LVO in the anterior circulation when performed within 6 hours of symptom onset [ 10, 14, 15, 27, 36, 63, 90]. The time interval since symptom onset was identified as the most crucial factor for determining the optimal management of stroke patients. The HERMES (Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke) study, a meta-analysis of five RCTs, similarly concluded that performing EVT after 7.3 hours did not confer any additional clinical benefit [ 91]. In the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) study, every hour of delay in reperfusion resulted in a 7.7% decreased probability of achieving functional independence [ 67]. Likewise, in the REVASCAT (Randomized Trial of Revascularization with Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset) study, every 30-minute delay in reperfusion resulted in a 26% decrease in the likelihood of a favorable outcome [ 79]. Consequently, streamlining workflow to reduce time delays has become the cornerstone of stroke systems for both intravenous and intraarterial therapies. The concept of penumbra, consisting of cells incapacitated due to lack of oxygen, has led to efforts to identify ischemic penumbra. In 2011, a multicenter study of 237 patients with anterior circulation LVO, treated beyond 8 hours of symptom onset based on perfusion imaging, showed good outcomes with comparable complication rates to those observed in early time window studies [ 38]. The DEFUSE (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution) 2 study in 2012 demonstrated comparable good outcomes after EVT in patients presenting within and beyond 6 hours when a target mismatch was noticed on perfusion imaging [ 48]. Efforts to identify patients with salvageable brain tissue even in the late time window beyond 6 hours led to the design of the DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) and DEFUSE 3 trials, which resulted in a paradigm shift from “time is brain” to “imaging is brain” [ 76]. The DAWN trial selected patients presenting between 6 and 24 hours based on severe clinical deficits (high National Institutes of Health stroke scale [NIHSS]) with a small established infarction (small core). This clinical-core mismatch successfully identified patients who would benefit from EVT in the late time window, and the trial was halted after a pre-specified interim analysis. The rate of functional independence at 90 days was 49% in the EVT group compared to 13% in the control group [ 72]. The DEFUSE 3 trial enrolled patients in the late time window (6-16 hours) with evidence of salvageable tissue on perfusion imaging. Following the presentation of the DAWN trial results in May 2017, the DEFUSE 3 trial was stopped due to lack of equipoise, and an early interim analysis confirmed the benefit of EVT over medical therapy in patients selected based on a tissue paradigm. Good outcomes in the treatment arm were 45%, compared to 17% in the medical arm [ 3].
CURRENT ISSUES OF EVT
Current advancements have been made in two key area : first, determining the expandability of EVT to benefit a larger number of eligible AIS, and second, improving the execution of EVT techniques for better clinical outcome.
Expanding eligibility for endovascular therapy
A significant number of patients are not receiving the potential benefits of this highly effective treatment, as less than 20% of patients with AIS currently undergo EVT. However, with the expansion of treatment eligibility criteria, this percentage could potentially increase to 43.1% [ 31]. The current guidelines, which are based on high-evidence level studies, employ restrictive eligibility criteria to optimize the chances of demonstrating the efficacy of EVT. Meanwhile, these criteria also exclude a substantial proportion of potentially eligible patients, thereby limiting the broader application of endovascular treatment. Although there are ongoing RCTs aimed at expanding the eligibility criteria for EVT, it remains challenging to address various aspects of eligibility in clinical trials due to financial issues, challenges in patient enrollment, and the difficulty of verifying statistical differences. However, there has been a growing number of observational studies supporting the off-label application of EVT, which further emphasizes the need to consider a broader population for this treatment.
Fig. 1 shows the timetable of RCTs regarding expanding the eligibility of EVT in AIS patients. Based on the number needed to treat (NNT) for achieving 90-day functional independence in severe RCTs, the population with expanded indications also demonstrated a meaningful response to EVT. Narrowing patient selection with precise criteria tends to improve treatment effectiveness ( Fig. 2).
EVT for large core infarction (LCI)
LCI is a common exclusion criterion in 21% of ineligible LVO patients [ 23]. Majority of early time window trials set a threshold for LCI, typically defined as an infarct volume of 50-70 mL or an Alberta Stroke Program Early CT Score (ASPECTS) of 6 [ 14, 83, 106]. The in-hospital metric for triaging patients with AIS is designed to exclude patients with a LCI, as they are considered at a higher risk of complications, including reperfusion injury following recanalization and an increased incidence of post-thrombectomy symptomatic intracerebral hemorrhage (sICH). Recent studies have suggested that an ASPECTS below 2 may result in futility, indicating limited potential benefit from EVT [ 33, 110]. Additionally, other studies have demonstrated a lack of benefit when the computed tomography perfusion (CTP)-determined core volume exceeds 100-150 mL [ 16, 85]. Meanwhile, multiple studies have suggested that performing EVT on a relatively large core profile, even in patients with baseline ASPECTS scores of 3-5 which were previously considered indicative of treatment futility, can offer meaningful treatment benefits and reduce disability and mortality [ 24, 81, 105]. There are issues regarding the precise measurement of the extent of ischemic change, particularly due to the absence of an available gold standard for ischemic core imaging. Predicting the core infarct using computed tomography (CT) or magnetic resonance imaging (MRI) is not completely correlated with tissue death [ 56]. Different cells, including neurons, are destined to be affected by ischemia in varying ways. The analysis of pooled individual patient data from the HERMES collaboration found that patients who underwent reperfusion had significantly better functional outcomes compared to those who did not undergo reperfusion, even when controlling for follow-up infarct volume [ 12]. Moreover, seemingly irreversible changes in MRI diffusion-weighted imaging (DWI) and non-contrast CT can potentially reverse after the restoration of blood flow [ 45]. As the time window increases, there is a noticeable decrease in the mismatch ratio, which correlates with a reduced benefit from EVT [ 95]. However, patients with a LCI who present in the early time window, often referred to as fast progressors, may still experience benefits from EVT through reperfusion of the salvageable mismatch area [ 24, 105]. Nonetheless, identifying the progressively decreasing mismatch profile in the early time window is challenging due to the limited time window and the poor predictive value of perfusion imaging in hyperacute window [ 16]. Recently, starting from 2022, three RCTs including RESCUE-Japan LIMIT (Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan Large Ischemic Core trial), ANGEL-ASPECT (Endovascular therapy in acute anterior circulation large vessel occlusive patients with a large infarct core), and SELECT 2 (Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke), have been published sequentially [ 35, 84, 100]. These trials have shown the potential for expanding the inclusion criteria for EVT to include patients with LCI ( Fig. 3). The first published RESCUE-Japan LIMIT study randomly assigned 203 patients with LCI, meeting the inclusion criteria of CT or DWI ASPECTS of 3-5 within 6 hours after the last known well or within 24 hours in the absence of early changes on fluid-attenuated inversion recovery (FLAIR) images. The study demonstrated improved functional outcomes, with a lower modified Rankin scale (mRS) score of 0 to 3 at 90 days in the EVT group compared to the medical care group (31.0% vs. 12.7%, relative risk 2.43; 95% confidence interval [CI], 1.35 to 4.37; p=0.002) [ 100]. The ANGEL-ASPECT study, conducted in China, randomly assigned 456 patients with LCI who met the inclusion criteria of ASPECTS of 3-5 or infarct core volume of 70-100 mL within 24 hours after the last known well. The study demonstrated a shift in the distribution of functional outcome at 90 days, with a generalized odds ratio of 1.37 (95% CI, 1.11 to 1.69; p=0.004), indicating a favorable treatment effect in the EVT group. However, it is important to note that the EVT group had a higher incidence of intracranial hemorrhage, with 6.1% experiencing sICH compared to 2.7% in the control group, and 49.1% experiencing any intracranial hemorrhage compared to 17.3% in the control group [ 35]. The SELECT 2 study randomly assigned 352 patients with similar inclusion criteria as the ANGEL-ASPECT study, except it included an infarct core volume of 50-70 mL. It demonstrated a better functional outcome with a generalized odds ratio of 1.51 (95% CI, 1.20 to 1.89; p<0.001). Interestingly, cerebral hemorrhage was infrequent in both groups [ 84]. Still ongoing RCTs, including the TENSION (Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window; NCT03094715), TESLA (Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke; NCT03805308), and LASTE (Large Stroke Therapy Evaluation; NCT03811769) trials, have varying inclusion criteria based on different definitions of large core volume infarct, imaging modalities, time windows, and the use of perfusion imaging ( Fig. 3). As the results of RCTs on LCI continue to emerge, it will be possible that we may witness not only an expansion of eligibility criteria for patients with LCI but also a fundamental change in the metrics used for patient selection.
EVT for strokes beyond 24 hours
Recent studies have primarily focused on a time window limited to 24 hours, and conducting prospective studies for AIS beyond 24 hours, including RCTs, poses challenges due to the small fraction of the AIS population. Patients with good collateral circulation may still exhibit a viable ischemic penumbra even in the later stages of stroke [ 19, 87]. Among these slow progressors, some fortunate individuals never experience further infarction progression, while the majority of non-reperfused patients typically encounter infarct progression within 3 days [ 47, 102]. About 20% of patients with LVO who present beyond 24 hours show persistent mismatch profile [ 22]. It appears that patients presenting beyond 24 hours, who otherwise meet DAWN or DEFUSE 3 criteria, may be safely treated with comparable clinical outcomes to those treated within 24 hours [ 1, 17, 19, 22]. In the propensity score-matched analyses using retrospective data of 150 patients with anterior circulation LVO with moderate to severe neurologic deficits persisting beyond 16 hours, about one-third of LVO patients met the inclusion criteria of either DAWN, DEFUSE 3, or ESCAPE (Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke) trials with identified salvageable tissues. EVT was performed in 16% of patients and showed better odds of good functional outcomes with 11.08-fold higher odds ratio [ 41]. In the SELECT LATE study, retrospective observational cohort study, 81% of patients who underwent EVT beyond 24 hours demonstrated the presence of a mismatch. These patients were more likely to achieve functional independence and had lower mortality rates. However, it should be noted that as time progressed, there was an increased tendency for hemorrhage to occur [ 86].
EVT for mild strokes with large penumbra
Clinical trials typically exclude patients with mild clinical stroke severity, defined as NIHSS scores ≤5, who are considered “too good to treat”. A population-based study of AIS indicates that approximately 7% of patients with LVO were not treated due to their mild symptoms [ 31]. Interestingly, some reports have found that 27-35% of AIS patients with low NIHSS scores still experience poor clinical outcomes despite receiving optimal medical management [ 9, 25, 39, 96]. Moreover, conservative management in 14.6% to 34.6% of patients with mild disability and LVO AIS can lead to frequent early neurologic deterioration (END) [ 42, 51, 78, 82]. In such cases, anticipative thrombectomy has been associated with improved clinical outcomes, while patients who undergo rescue thrombectomy after experiencing END have shown worse outcomes compared to those who receive either optimal medical management alone or immediate EVT alone (54.5% rescue EVT, 71.7% optimal medical management alone, 85% immediate EVT; p=0.007) [ 69]. However, another study argues that the potential risk associated with neurologic deterioration or hemorrhagic event after EVT might outweigh the risk of END after optimal medical management [ 101]. Not all patients with low NIHSS scores are equivalent in terms of clinical outcomes, highlighting the limitations of using NIHSS as a screening tool for treatment eligibility. The heterogeneity of clinical outcomes emphasizes the need for better methods of patient selection. While guidelines emphasize the importance of disabling symptoms in patient selection for IVT, there is currently no evidence supporting the differentiation of patients with low NIHSS scores based on the presence or absence of disabling symptoms [ 40, 93]. More proximal LVO and longer thrombus length are known predictive factors of END [ 65, 94], while hypoperfusion severity, such as the volume of Tmax >10 seconds or the hypoperfusion intensity ratio (HIR), is another candidate for predicting END [ 21, 30]. The upcoming RCTs, ENDOLOW (Endovascular therapy for low NIHSS ischemic stroke; NCT04167527) and MOSTE (Minor Stroke Therapy Evaluation; NCT03796468), are expected to shed light on the benefits of EVT compared to medical management.
EVT for distal and medium vessel occlusion (DMVO)
DMVOs constitute 25-40% of AIS [ 89]. With advances in catheter technology, the development of smaller thrombectomy devices, and increasing expertise in neuroendovascular procedures, DMVOs have been identified as a potential frontier in the EVT of AIS. However, there are disagreements regarding the use of EVT for DMVO, primarily due to concerns about the procedural safety of performing EVT in small-calibered vessels. Additionally, it has been observed that more distal occlusions tend to exhibit a better response to IVT. Nevertheless, IVT fails to achieve successful recanalization in 50% of DMVOs [ 11, 77]. Moreover, dealing with secondary DMVOs after primary intra-arterial or intravenous procedures is becoming a matter of concern as EVT becomes more widespread. It is worth noting that currently, no further RCTs have reported on expanding the indications for DMVOs. Efforts to expand the indications of EVT for more distal vessel occlusions began with M2 occlusion [ 88]. Distinguishing M2 occlusion from M1 occlusion can be challenging due to anatomical variations. However, an analysis of patients with M2 segment occlusion from the HERMES collaboration demonstrated that EVT resulted in improved functional outcomes for individuals with M2 segment occlusion [ 61]. Importantly, this improvement was achieved without an increased risk of complications related to the treatment. The 2019 guidelines from the Society for Neuro-interventional Surgery have issued a class I, level A recommendation for thrombectomy in the middle cerebral artery-M2 location [ 64]. For more distal occlusions, the TOPMOST (Treatment for primary distal, medium vessel occlusion stroke) study, a case-control study involving 184 patients with distal occlusion of the posterior cerebral artery (PCA), demonstrated the clinical benefit, feasibility, and safety of the endovascular technique for treating such occlusions [ 62]. However, apart from this clinical trial of EVT for PCA occlusion, the issue regarding occlusions in locations even more distal than the M2 segment remains unclear. The current EVT technique for LVO is mainly focused on how to effectively access tortuous distal vessels and reduce thrombus fragmentation. However, the fragility of distal and medium intracranial vessels, characterized by smaller caliber and thinner arterial walls compared to proximal large vessels, hesitates the application of the same techniques used for EVT in LVO due to hemorrhagic complications and thrombectomy-related vessel injury. A combined strategy, mediated by blind exchange mini-pinning (BEMP), utilizing a low-profile mini aspiration catheter and a micro-stent retriever for thrombectomy in small-calibered vessels, has been proposed to achieve a higher rate of recanalization while reducing the incidence of hemorrhagic events and vessel injury [ 34]. However, given that symptoms in patients with DMVOs tend to be mild, careful patient selection should be ensured.
EVT for posterior circulation strokes
Posterior circulation LVO comprises 5% of all LVOs [ 23]. With devastating nature of posterior circulation strokes, the rate of mortality or severe morbidity was reported up to 90% [ 53]. According to prospective registries of EVT for acute basilar artery occlusion (BAO), although the successful reperfusion rate after EVT is comparable to that of anterior circulation LVO, only about 27.4% of patients experience favorable outcomes, indicating a high rate of futile recanalization [ 104]. Until 2022, the endeavor to establish the superiority of EVT over medical treatment in acute BAO faced a setback with the neutral results from two RCTs. These trials enrolled patients with BAO who presented within the 6-hour window in the BASICS (Basilar Artery International Cooperation Study) trial and within the 8-hour window in the BEST (Basilar Artery Occlusion Endovascular Intervention Versus Standard Medical Treatment) trial. Experts have pointed out that the study design of both RCTs has pitfalls, including high rates of crossovers in the BEST trial and a lack of consecutive enrollment in BASICS [ 46, 54]. According to subgroup analysis of the BASICS trial and previous observational studies, which indicated that patients with minor deficits (NIHSS <10) [ 46, 92] and extensive baseline infarction or large pontine infarct do not derive significant benefits from EVT compared to medical therapy [ 4, 59, 107], the eligibility criteria of other ongoing RCTs, the ATTENTION (Endovascular Treatment for Acute Basilar Artery Occlusion trial) and BAOCHE (Basilar Artery Occlusion Chinese Endovascular) trial, have undergone refinement, resulting in narrower criteria. The ATTENTION trial randomized 340 patients with BAO who presented within 12 hours of the estimated time (i.e., onset of severe symptoms or coma) of BAO, had moderate to severe symptoms defined by an NIHSS score of ≥10, and a posterior circulation ASPECTS (pc-ASPECTS) of ≥6 (or ≥8 in patients ≥80 years) [ 98]. On the other hand, the BAOCHE trial recruited 217 patients with BAO who presented from 6 to 24 hours since symptom onset. Although the BAOCHE trial included patients with an pc-ASPECTS score ≥6 and ponsmidbrain index of ≤2, the majority of patients included had an NIHSS score higher than 10 [ 37]. Given the anticipated dismal prognosis of BAO, the primary outcome in both trials was defined as achieving a mRS of 0-3. In both trials, EVT demonstrated improved functional outcomes compared with medical therapy. The ATTENTION trial showed higher rates of favorable functional outcomes, with an mRS score of 0-3 at 90 days in the EVT group compared to the medical management group (46% vs. 23%; relative risk, 2.06; 95% CI, 1.46 to 2.91; p<0.001) [ 98]. Similarly, in the BAOCHE trial, which included patients in the late time window without advanced imaging assessment, similar functional outcomes were noted for EVT (46% vs. 24%; relative risk, 1.81; 95% CI, 1.26 to 2.60; p<0.001) [ 37]. Notably, the rates of symptomatic hemorrhage in both trials were comparable to the reports of anterior circulation LVO [ 37, 98]. Therefore, EVT appears to be beneficial in patients with BAO who present with moderate to severe symptoms.
Assessing the perfection and effectiveness
Angiographic outcomes after EVT have shown a tendency toward greater success. According to an early period study, the reported overall successful recanalization rate was 70.5% [ 28]. In a recent trial, both the contact aspiration and stent retriever groups demonstrated higher rates of TICI ≥2b, exceeding 80-90% [ 50, 99]. Furthermore, multiple combined techniques with minor variations have been updated, leading to nearly 100% successful reperfusion rates [ 57, 103]. Achieving complete or near-complete reperfusion has been linked to better clinical outcomes and fewer adverse effects. Meanwhile, the proliferation of numerous variations and hybridizations of the technique has made it challenging for operators to select the appropriate technique for specific situations [ 68]. However, despite advancements in EVT techniques and increased expertise, complete reperfusion is only achieved in approximately half of cases, often necessitating multiple attempts and the use of rescue therapy. Moreover, the level of difficulty in performing EVT could be elevated by the underlying pathology of LVO.
Chronology of target for EVT
The extent of reperfusion achieved is among the few but most critical modifiable determinants for better clinical outcomes. The higher the amount of the reperfused tissue, and the lower the chance of penumbra evolving into infarction.
The modified Thrombolysis In Cerebral Infarction (mTICI) score, introduced in 2013, has been the main parameter for assessing angiographic outcomes, with most landmark studies demonstrating the superiority of EVT over medical treatment for LVO of AIS patients defining mTICI 2b or 3 as successful reperfusion for EVT [ 111]. Consequently, the target angiographic endpoint has been set to mTICI 2b, although further attempts for better reperfusion could be made. Predicting clinical outcomes concisely with mTICI 2b-3 is challenging due to the heterogeneity of the damaged volume in the cerebral hemisphere. A subsequent expanded TICI (eTICI) grade, which subdivides categories more finely into seven groups, has been shown to correlate incrementally with clinical outcomes [ 52]. With meaningful differences in clinical outcomes between mTICI 3 and mTICI 2b, the old-fashioned definition of successful reperfusion confronts its clinical efficiency [ 20, 29, 44]. This implies that maximal effect for reperfusion is desirable for clinical outcomes after EVT. Recently published studies have aimed to set successful reperfusion as eTICI 3 or 2c [ 80]. Additionally, achieving the best possible reperfusion with a single passage is considered another treatment goal of EVT. Maximal reperfusion after multiple attempts with passage of devices versus achieving maximal reperfusion with a single attempt of EVT seem to be two different scenarios. The first-pass effect (FPE), defined as complete revascularization (eTICI2c-3) following a single passage of the device without the need for rescue therapy, has been applied as a metric of mechanical thrombectomy success [ 108]. While it is controversial whether FPE is a major factor contributing to favorable outcomes or merely an epiphenomenon, studies have reported that FPE is an independent predictor of good clinical outcomes, regardless of procedural time. A matched case-control study, which controlled for onset-to-reperfusion time between single and multiple passages, has demonstrated that FPE improves clinical outcomes by reducing the likelihood of radiographically negative small embolic infarctions and periprocedural vessel injury, regardless of procedural time or duration of ischemic time [ 71, 108]. Even among patients who achieve total recanalization after EVT, the presence or absence of FPE could still make a difference in clinical outcomes [ 8, 71].
Debates regarding techniques of EVT
Numerous EVT techniques have emerged to improve the speed and quality of reperfusion, thereby increasing the likelihood of positive outcomes. Comparing the contact aspiration technique versus the stent retriever as a front-line technique may be somewhat outdated, given the variety of thrombectomy devices now frequently used in combination with advanced techniques.
Using direct aspiration in its infancy confronted the drawbacks of lacking catheters with a large enough diameter for sufficient aspiration and an absence of catheters with enough flexibility and atraumatic feature to navigate the tortuous intracranial vasculature. Therefore, at that time, the stent retriever technique became the predominant method for EVT, following landmark RCTs in 2015 [ 10, 14, 15, 27, 63, 75, 90]. But, with the availability of large-bore catheters that offer improved distal trackability, the contact aspiration technique, also known as A Direct Aspiration First Pass Technique (ADAPT), has gained favor. Subsequently, two RCTs, ASTER (The Contact Aspiration vs Stent Retriever for Successful Revascularization) and COMPASS (Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion) trials, demonstrated similar procedural and clinical outcomes between the contact aspiration and stent retriever techniques [ 50, 99]. After demonstrating the non-inferiority of aspiration thrombectomy in the COMPASS trial, subsequent studies have focused on determining which techniques are superior based on individual patient and clot characteristics. However, the preference for first-line techniques is still influenced by the facility and the interventionist [ 58]. Efforts to enhance the likelihood of successful reperfusion have led to combination techniques involving stent retrievers and large-bore aspiration catheters. When combined with proximal aspiration or flow arrest using a balloon guiding catheter, the technique commonly known as the Solumbra technique has undergone significant modifications and adaptations, resulting in heterogeneity in its application and outcomes [ 68]. Although reported rates of successful reperfusion (TICI ≥2b) have reached nearly 100%, this has resulted in a low level of evidence for each individual technique based on single-center experiences [ 57, 103]. The ASTER2 trial, a RCT comparing the concomitant use of direct aspiration and stent retriever versus stent retriever alone, failed to demonstrate an improved rate of near-total or total reperfusion (eTICI2c/3) at the end of the EVT using combined technique. However, aspects such as a higher FPE, a lower number of attempts, and a lower rate of distal embolization associated with the usage of the combined technique are encouraging [ 49]. Considering the first-line thrombectomy technique for the posterior circulation, recent studies suggest that contact aspiration may result in better clinical outcomes compared to the stent retriever, contrary to the leveled outcomes observed in anterior circulation EVT [ 26, 55]. The STAR collaboration, which compared the effects between the combined technique and direct aspiration alone, showed that first-line contact aspiration could achieve better reperfusion and clinical outcomes than the combined techniques [ 2]. The ongoing pc-ASTER trial (NCT05320263) aims to elucidate which technique, either contact aspiration or stent retriever, is better for the treatment of BAO. The question of how to achieve the best angiographic results remains unanswered. Despite numerous inconclusive and contradictory reports regarding the superiority of different EVT techniques, it is important to reflect on the changing paradigm of the thrombectomy goal.
Rescue strategies for formidable cases of EVT
While EVT has shown promising results for many AIS patients, certain subsets face challenges in deriving benefits from it. Notably, cases involving underlying intracranial atherosclerotic stenosis (ICAS) represent formidable obstacles. ICAS has been identified as a prevalent cause of LVO, particularly among Asian and African American populations [ 97]. Detecting ICAS before undergoing EVT can be particularly challenging, especially in patients lacking clear angiographic clues, such as intracranial vessel calcification, concurrent ICAS in other intracranial vessels, fluctuating symptom presentations, or a well-established preprocedural leptomeningeal collateral status [ 7, 13]. A substantial proportion of ICAS-related LVO cases require multiple passages, with or without reperfusion, leading to delayed reperfusion times and cumulative risks of procedural complications. Skepticism regarding the use of empirical angioplasty or stenting for reperfused ICAS-related LVO (when TICI ≥2b has been maintained) for the preventive purpose of reocclusion is based on concerns about the potentially high rate of periprocedural complications [ 18, 66, 73, 109]. Meanwhile, in clinical practice, instances of failed reperfusion following multiple attempts with conventional EVT techniques call for the development of contingency plans that involve alternative methods. These rescue techniques encompass balloon angioplasty, intraarterial thrombolytics with glycoprotein IIb/IIIa inhibitors, intracranial stenting, and various combinations of these techniques [ 43, 81]. Limited evidence, primarily from a few observational studies conducted mainly in Asian populations, has reported positive effects on target vessel patency and improved functional outcomes, all without an increase in intracranial hemorrhage or mortality rates [ 6, 74]. When comparing the effectiveness of different rescue strategies, it was observed that the combination of rescue stenting and thrombolytic infusion resulted in a better reperfusion rate and more favorable outcomes compared to using a single rescue modality [ 5]. Ongoing ANGEL REBOOT (Randomised study of bailout intracranial angioplasty following thrombectomy for acute large vessel occlusion; NCT05122286) aims to assess the efficacy and safety of rescue intracranial stenting following failed EVT in acute AIS LVO, regardless of the underlying etiology of the stroke.
CONCLUSION
Based on positive high-quality data, justifying the expanded application of EVT for AIS to a wider population through guideline changes seems reasonable. Optimizing reperfusion and minimizing the number of passages during endovascular procedures are key objectives for improving clinical outcomes. Despite institutional or provider variability in procedural techniques, efforts to investigate controversial issues should continue.
Fig. 1.
Chronology in the history of randomized controlled trials investigating eligibility of endovascular thrombectomy in acute ischemic stroke patients. NINDS : National Institute of Neurological Disorders and Stroke, IV tPA : intravenous tissue plasminogen activator, PROACT : Prolyse in Acute Cerebral Thromboembolism, IA : intra-arterial, ECASS : European Cooperative Acute Stroke Study trial, SYNTHESIS : Local versus Systemic Throm-bolysis for Acute Ischemic Stroke, IMS : Interventional Management of Stroke, MR RESCUE : Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy, EXTEND IA : Extending the Time for Thrombolysis in Emergency Neurological Deficits - Intra-Arterial, MR CLEAN : Multicenter Randomized Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands, SWIFT PRIME : Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment, REVASCAT : Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset, ESCAPE : Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke, HERMES : Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke, DAWN : DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo, DEFUSE : Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke, RESCUE-Japan LIMIT : Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan Large Ischemic Core Trial, ANGEL-ASPECT : Endovascular therapy in acute anterior circulation large vessel occlusive patients with a large infarct core, SELECT 2 : Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke, TENSION : Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window, TESLA : Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke, LASTE : Large Stroke Therapy Evaluation, BEST : Basilar Artery Occlusion Endovascular Intervention Versus Standard Medical Treatment, BASICS : Basilar Artery International Cooperation Study, ATTENTION : Endovascular Treatment for Acute Basilar Artery Occlusion trial, BAOCHE : Basilar Artery Occlusion Chinese Endovascular, ENDOLOW : Endovascular therapy for low NIHSS ischemic stroke, MOSTE : Minor Stroke Therapy Evaluation. 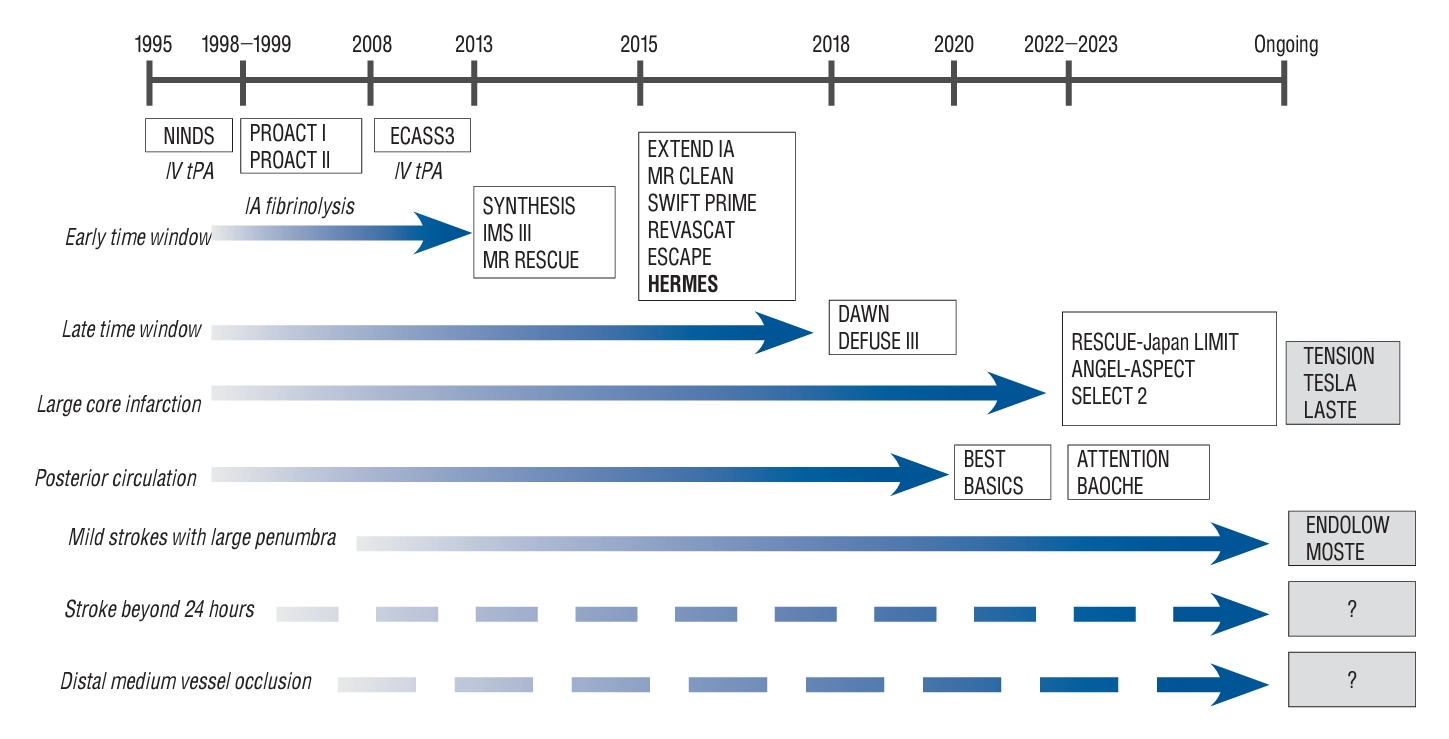
Fig. 2.
The number needed to treat for randomized controlled trials of endovascular thrombectomy in acute ischemic stroke patients. NINDS : National Institute of Neurological Disorders and Stroke, ECASS : European Cooperative Acute Stroke Study trial, MR CLEAN : Multicenter Randomized Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands, THRACE : Mechanical Thrombectomy After Intravenous Alteplase Versus Alteplase Alone After Stroke, REVASCAT : Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset, ESCAPE : Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke, EXTEND IA : Extending the Time for Thrombolysis in Emergency Neurological De cits - Intra-Arterial, SWIFT PRIME : Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment, HERMES : Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke, DAWN : DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo, DEFUSE : Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke, RESCUE-Japan : Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan, ANGEL-ASPECT : Endovascular therapy in acute anterior circulation large vessel occlusive patients with a large infarct core, SELECT 2 : Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke, BEST : Basilar Artery Occlusion Endovascular Intervention Versus Standard Medical Treatment, BASICS : Basilar Artery International Cooperation Study, ATTENTION : Endovascular Treatment for Acute Basilar Artery Occlusion trial, BAOCHE : Basilar Artery Occlusion Chinese Endovascular, IV tPA : intravenous tissue plasminogen activator, EVT : endovascular thrombectomy. 
Fig. 3.
Inclusion criteria of randomized controlled trials of large core infarction. MR : magnetic resonance, ASPECTS : Alberta Stroke Program Early Computed Tomography Score, CT : computed tomography, SELECT 2 : Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke, ANGEL-ASPECT : Endovascular therapy in acute anterior circulation large vessel occlusive patients with a large infarct core, LASTE : Large Stroke Therapy Evaluation, TESLA : Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke, RESCUE-Japan LIMIT : Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan Large Ischemic Core trial, TENSION : Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window, EVT : endovascular thrombectomy. 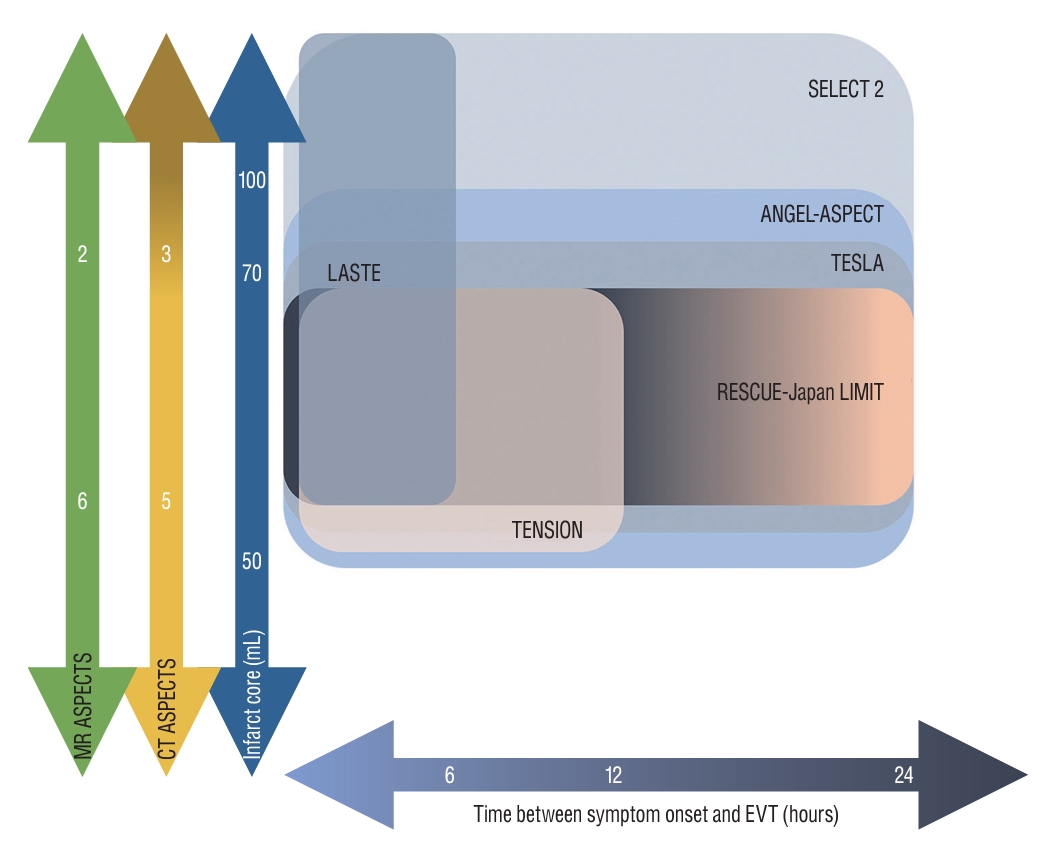
References
1. Aguilar-Salinas P, Santos R, Granja MF, Effendi S, Sauvageau E, Hanel R, et al : Republished: revisiting the therapeutic time window dogma: successful thrombectomy 6 days after stroke onset. J Neurointerv Surg 11 : e82019   2. Alawieh AM, Eid M, Anadani M, Sattur M, Maier IL, Feng W, et al : Thrombectomy technique predicts outcome in posterior circulation stroke-insights from the STAR collaboration. Neurosurgery 87 : 982-991, 2020    3. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al : Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378 : 708-718, 2018   4. Alexandre AM, Valente I, Consoli A, Piano M, Renieri L, Gabrieli JD, et al : Posterior circulation endovascular thrombectomy for large-vessel occlusion: predictors of favorable clinical outcome and analysis of first-pass effect. AJNR Am J Neuroradiol 42 : 896-903, 2021    5. Baek JH, Jung C, Kim BM, Heo JH, Kim DJ, Nam HS, et al : Combination of rescue stenting and antiplatelet infusion improved outcomes for acute intracranial atherosclerosis-related large-vessel occlusion. Front Neurol 12 : 608270, 2021    6. Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Yoo J : Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke 47 : 2360-2363, 2016   7. Baek JH, Kim BM, Kim JW, Kim DJ, Heo JH, Nam HS, et al : Utility of leptomeningeal collaterals in predicting intracranial atherosclerosis-related large vessel occlusion in endovascular treatment. J Clin Med 9 : 2784, 2020    8. Bai X, Zhang X, Yang W, Zhang Y, Wang T, Xu R, et al : Influence of first-pass effect on recanalization outcomes in the era of mechanical thrombectomy: a systemic review and meta-analysis. Neuroradiology 63 : 795-807, 2021    9. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM : Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 56 : 1015-1020, 2001   10. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372 : 11-20, 2015  11. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al : Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41 : 2254-2258, 2010   12. Boers AMM, Jansen IGH, Brown S, Lingsma HF, Beenen LFM, Devlin TG, et al : Mediation of the relationship between endovascular therapy and functional outcome by follow-up infarct volume in patients with acute ischemic stroke. JAMA Neurol 76 : 194-202, 2019   13. Bos D, Portegies ML, van der Lugt A, Bos MJ, Koudstaal PJ, Hofman A, et al : Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam study. JAMA Neurol 71 : 405-411, 2014   14. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al : Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 15 : 1138-1147, 2016   15. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al : Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372 : 1009-1018, 2015  16. Campbell BCV, Majoie CBLM, Albers GW, Menon BK, Yassi N, Sharma G, et al : Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 18 : 46-55, 2019  17. Casetta I, Fainardi E, Pracucci G, Saia V, Vallone S, Zini A, et al : Endovascular treatment beyond 24 hours from the onset of acute ischemic stroke: the Italian Registry of Endovascular Thrombectomy in Acute Stroke (IRETAS). J Neurointerv Surg 14 : 1186-1188, 2022   18. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al : Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 365 : 993-1003, 2011   19. Christensen S, Mlynash M, Kemp S, Yennu A, Heit JJ, Marks MP, et al : Persistent target mismatch profile >24 hours after stroke onset in DEFUSE 3. Stroke 50 : 754-757, 2019    20. Dargazanli C, Consoli A, Barral M, Labreuche J, Redjem H, Ciccio G, et al : Impact of modified TICI 3 versus modified TICI 2b reperfusion score to predict good outcome following endovascular therapy. AJNR Am J Neuroradiol 38 : 90-96, 2017    21. de Havenon A, Mlynash M, Kim-Tenser MA, Lansberg MG, Leslie-Mazwi T, Christensen S, et al : Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke 50 : 632-638, 2019    22. Desai SM, Haussen DC, Aghaebrahim A, Al-Bayati AR, Santos R, Nogueira RG, et al : Thrombectomy 24 hours after stroke: beyond DAWN. J Neurointerv Surg 10 : 1039-1042, 2018   23. Desai SM, Starr M, Molyneaux BJ, Rocha M, Jovin TG, Jadhav AP : Acute ischemic stroke with vessel occlusion-prevalence and thrombectomy eligibility at a comprehensive stroke center. J Stroke Cerebrovasc Dis 28 : 104315, 2019   24. Gautheron V, Xie Y, Tisserand M, Raoult H, Soize S, Naggara O, et al : Outcome after reperfusion therapies in patients with large baseline diffusion-weighted imaging stroke lesions: a THRACE trial (mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke) subgroup analysis. Stroke 49 : 750-753, 2018   25. Goldhoorn RB, Mulder MJHL, Jansen IGH, van Zwam WH, Staals J, van der Lugt A, et al : Safety and outcome of endovascular treatment for minor ischemic stroke: results from the multicenter clinical registry of endovascular treatment of acute ischemic stroke in the Netherlands. J Stroke Cerebrovasc Dis 28 : 542-549, 2019   26. Gory B, Mazighi M, Blanc R, Labreuche J, Piotin M, Turjman F, et al : Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first-line strategy (ADAPT vs stent retriever). J Neurosurg 129 : 1482-1491, 2018   27. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al : Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372 : 1019-1030, 2015  28. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al : Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387 : 1723-1731, 2016   29. Goyal N, Tsivgoulis G, Frei D, Turk A, Baxter B, Froehler MT, et al : Comparative safety and efficacy of modified TICI 2b and TICI 3 reperfusion in acute ischemic strokes treated with mechanical thrombectomy. Neurosurgery 84 : 680-686, 2019   30. Guenego A, Mlynash M, Christensen S, Kemp S, Heit JJ, Lansberg MG, et al : Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol 84 : 616-620, 2018    31. Guisado-Alonso D, Martínez-Domeño A, Prats-Sánchez L, Delgado-Mederos R, Camps-Renom P, Abilleira S, et al : Reasons for not performing mechanical thrombectomy: a population-based study of stroke codes. Stroke 52 : 2746-2753, 2021   32. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al : Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359 : 1317-1329, 2008   33. Han M, Choi JW, Rim NJ, Kim SY, Suh HI, Lee KS, et al : Cerebral infarct volume measurements to improve patient selection for endovascular treatment. Medicine (Baltimore) 95 : e47022016    34. Haussen DC, Al-Bayati AR, Eby B, Ravindran K, Rodrigues GM, Frankel MR, et al : Blind exchange with mini-pinning technique for distal occlusion thrombectomy. J Neurointerv Surg 12 : 392-395, 2020   35. Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, et al : Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med 388 : 1272-1283, 2023  36. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al : Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372 : 2296-2306, 2015   37. Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, et al : Trial of thrombectomy 6 to 24 hours after stroke due to Basilar-Artery occlusion. N Engl J Med 387 : 1373-1384, 2022  38. Jovin TG, Liebeskind DS, Gupta R, Rymer M, Rai A, Zaidat OO, et al : Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: retrospective multicenter analysis of 237 consecutive patients. Stroke 42 : 2206-2211, 2011   39. Kenmuir CL, Hammer M, Jovin T, Reddy V, Wechsler L, Jadhav A : Predictors of outcome in patients presenting with acute ischemic stroke and mild stroke scale scores. J Stroke Cerebrovasc Dis 24 : 1685-1689, 2015   40. Khatri P, Kleindorfer DO, Devlin T, Sawyer RN Jr, Starr M, Mejilla J, et al : Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA 320 : 156-166, 2018   41. Kim BJ, Menon BK, Kim JY, Shin DW, Baik SH, Jung C, et al : Endovascular treatment after stroke due to large vessel occlusion for patients presenting very late from time last known well. JAMA Neurol 78 : 21-29, 2020    42. Kim JT, Park MS, Chang J, Lee JS, Choi KH, Cho KH : Proximal arterial occlusion in acute ischemic stroke with low NIHSS scores should not be considered as mild stroke. PLoS One 8 : e709962013    44. Kleine JF, Wunderlich S, Zimmer C, Kaesmacher J : Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg 9 : 117-121, 2017   45. Labeyrie MA, Turc G, Hess A, Hervo P, Mas JL, Meder JF, et al : Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke 43 : 2986-2991, 2012   46. Langezaal LCM, van der Hoeven EJRJ, Mont’Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al : Endovascular therapy for stroke due to Basilar-Artery occlusion. N Engl J Med 384 : 1910-1920, 2021   47. Lansberg MG, O’Brien MW, Tong DC, Moseley ME, Albers GW : Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch Neurol 58 : 613-617, 2001   48. Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al : MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 11 : 860-867, 2012    49. Lapergue B, Blanc R, Costalat V, Desal H, Saleme S, Spelle L, et al : Effect of thrombectomy with combined contact aspiration and stent retriever vs stent retriever alone on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER2 randomized clinical trial. JAMA 326 : 1158-1169, 2021   50. Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, et al : Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA 318 : 443-452, 2017    51. Lee VH, Thakur G, Nimjee SM, Youssef PP, Lakhani S, Heaton S, et al : Early neurologic decline in acute ischemic stroke patients receiving thrombolysis with large vessel occlusion and mild deficits. J Neurointerv Surg 12 : 1085-1087, 2020   52. Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, et al : eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 11 : 433-438, 2019   53. Lindsberg PJ, Mattle HP : Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke 37 : 922-928, 2006   54. Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al : Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 19 : 115-122, 2020  55. Ma G, Sun X, Tong X, Jia B, Huo X, Luo G, et al : Safety and efficacy of direct angioplasty in acute basilar artery occlusion due to atherosclerosis. Front Neurol 12 : 651653, 2021    57. McTaggart RA, Tung EL, Yaghi S, Cutting SM, Hemendinger M, Gale HI, et al : Continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE): a technique which improves outcomes. J Neurointerv Surg 9 : 1154-1159, 2017   58. Mehta T, Male S, Quinn C, Kallmes DF, Siddiqui AH, Turk A, et al : Institutional and provider variations for mechanical thrombectomy in the treatment of acute ischemic stroke: a survey analysis. J Neurointerv Surg 11 : 884-890, 2019   59. Meinel TR, Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Mosimann PJ, et al : Mechanical thrombectomy for basilar artery occlusion: efficacy, outcomes, and futile recanalization in comparison with the anterior circulation. J Neurointerv Surg 11 : 1174-1180, 2019    60. Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al : Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA 320 : 1017-1026, 2018    61. Menon BK, Hill MD, Davalos A, Roos YBWEM, Campbell BCV, Dippel DWJ, et al : Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: meta-analysis of data from the HERMES Collaboration. J Neurointerv Surg 11 : 1065-1069, 2019   62. Meyer L, Stracke CP, Jungi N, Wallocha M, Broocks G, Sporns PB, et al : Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol 78 : 434-444, 2021  63. Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, et al : Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke 47 : 2331-2338, 2016   64. Mokin M, Ansari SA, McTaggart RA, Bulsara KR, Goyal M, Chen M, et al : Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS standards and guidelines committee. J Neurointerv Surg 11 : 215-220, 2019   65. Mokin M, Masud MW, Dumont TM, Ahmad G, Kass-Hout T, Snyder KV, et al : Outcomes in patients with acute ischemic stroke from proximal intracranial vessel occlusion and NIHSS score below 8. J Neurointerv Surg 6 : 413-417, 2014   66. Mosimann PJ, Kaesmacher J, Gautschi D, Bellwald S, Panos L, Piechowiak E, et al : Predictors of unexpected early reocclusion after successful mechanical thrombectomy in acute ischemic stroke patients. Stroke 49 : 2643-2651, 2018   67. Mulder MJHL, Jansen IGH, Goldhoorn RB, Venema E, Chalos V, Compagne KCJ, et al : Time to endovascular treatment and outcome in acute ischemic stroke: MR CLEAN registry results. Circulation 138 : 232-240, 2018   68. Munoz A, Jabre R, Orenday-Barraza JM, Eldin MS, Chen CJ, Al-Saiegh F, et al : A review of mechanical thrombectomy techniques for acute ischemic stroke. Interv Neuroradiol 29 : 450-458, 2023    69. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group : Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333 : 1581-1587, 1995   70. Nagel S, Bouslama M, Krause LU, Küpper C, Messer M, Petersen M, et al : Mechanical thrombectomy in patients with milder strokes and large vessel occlusions. Stroke 49 : 2391-2397, 2018   71. Nikoubashman O, Dekeyzer S, Riabikin A, Keulers A, Reich A, Mpotsaris A, et al : True first-pass effect. Stroke 50 : 2140-2146, 2019   72. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al : Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378 : 11-21, 2018  73. Oliveira R, Correia MA, Marto JP, Carvalho Dias M, Mohamed GA, Nguyen TN, et al : Reocclusion after successful endovascular treatment in acute ischemic stroke: systematic review and meta-analysis. J Neurointerv Surg 15 : 964-970, 2023   74. Peng F, Wan J, Liu W, Huang W, Wang L, Qiu T, et al : Efficacy and safety of rescue stenting following failed mechanical thrombectomy for anterior circulation large vessel occlusion: propensity score analysis. J Neurointerv Surg 12 : 271-273, 2020   75. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al : 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46 : 3020-3035, 2015  76. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al : 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49 : e46-e110, 2018  77. Rahme R, Yeatts SD, Abruzzo TA, Jimenez L, Fan L, Tomsick TA, et al : Early reperfusion and clinical outcomes in patients with M2 occlusion: pooled analysis of the PROACT II, IMS, and IMS II studies. J Neurosurg 121 : 1354-1358, 2014   78. Rajajee V, Kidwell C, Starkman S, Ovbiagele B, Alger JR, Villablanca P, et al : Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology 67 : 980-984, 2006   79. Ribo M, Molina CA, Cobo E, Cerdà N, Tomasello A, Quesada H, et al : Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke 47 : 999-1004, 2016  80. Rizvi A, Seyedsaadat SM, Murad MH, Brinjikji W, Fitzgerald ST, Kadirvel R, et al : Redefining ‘success’: a systematic review and meta-analysis comparing outcomes between incomplete and complete revascularization. J Neurointerv Surg 11 : 9-13, 2019   81. Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al : Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 17 : 895-904, 2018  82. Saleem Y, Nogueira RG, Rodrigues GM, Kim S, Sharashidze V, Frankel M, et al : Acute neurological deterioration in large vessel occlusions and mild symptoms managed medically. Stroke 51 : 1428-1434, 2020   83. Sanák D, Nosál’ V, Horák D, Bártková A, Zelenák K, Herzig R, et al : Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology 48 : 632-639, 2006    84. Sarraj A, Hassan AE, Abraham MG, Ortega-Gutierrez S, Kasner SE, Hussain MS, et al : Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med 388 : 1259-1271, 2023  85. Sarraj A, Hassan AE, Savitz S, Sitton C, Grotta J, Chen P, et al : Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient’s selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol 76 : 1147-1156, 2019    86. Sarraj A, Kleinig TJ, Hassan AE, Portela PC, Ortega-Gutierrez S, Abraham MG, et al : Association of endovascular thrombectomy vs medical management with functional and safety outcomes in patients treated beyond 24 hours of last known well: the SELECT late study. JAMA Neurol 80 : 172-182, 2023   87. Sarraj A, Mlynash M, Heit J, Pujara D, Lansberg M, Marks M, et al : Clinical outcomes and identification of patients with persistent penumbral profiles beyond 24 hours from last known well: analysis from DEFUSE 3. Stroke 52 : 838-849, 2021   88. Sarraj A, Sangha N, Hussain MS, Wisco D, Vora N, Elijovich L, et al : Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol 73 : 1291-1296, 2016   89. Saver JL, Chapot R, Agid R, Hassan A, Jadhav AP, Liebeskind DS, et al : Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 51 : 2872-2884, 2020   90. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372 : 2285-2295, 2015   91. Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al : Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 316 : 1279-1288, 2016  92. Schonewille WJ, Wijman CA, Michel P, Rueckert CM, Weimar C, Mattle HP, et al : Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 8 : 724-730, 2009   93. Schwarz G, Cascio Rizzo A, Matusevicius M, Giussani G, Invernizzi P, Melis F, et al : Reperfusion treatments in disabling versus nondisabling mild stroke due to anterior circulation vessel occlusion. Stroke 54 : 743-750, 2023   94. Seners P, Ben Hassen W, Lapergue B, Arquizan C, Heldner MR, Henon H, et al : Prediction of early neurological deterioration in individuals with minor stroke and large vessel occlusion intended for intravenous thrombolysis alone. JAMA Neurol 78 : 321-328, 2021  95. Seners P, Oppenheim C, Turc G, Albucher JF, Guenego A, Raposo N, et al : Perfusion imaging and clinical outcome in acute ischemic stroke with large core. Ann Neurol 90 : 417-427, 2021    96. Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH : Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke 36 : 2497-2499, 2005   97. Suri MF, Johnston SC : Epidemiology of intracranial stenosis. J Neuroimaging 19 Suppl 1 : 11S-16S, 2009  98. Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, et al : Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med 387 : 1361-1372, 2022  99. Turk AS 3rd, Siddiqui A, Fifi JT, De Leacy RA, Fiorella DJ, Gu E, et al : Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 393 : 998-1008, 2019   100. Uchida K, Shindo S, Yoshimura S, Toyoda K, Sakai N, Yamagami H, et al : Association between alberta stroke program early computed tomography score and efficacy and safety outcomes with endovascular therapy in patients with stroke from large-vessel occlusion: a secondary analysis of the recovery by endovascular salvage for cerebral ultra-acute embolism-japan large ischemic core trial (RESCUE-Japan LIMIT). JAMA Neurol 79 : 1260-1266, 2022   101. Volny O, Zerna C, Tomek A, Bar M, Rocek M, Padr R, et al : Thrombectomy vs medical management in low NIHSS acute anterior circulation stroke. Neurology 95 : e3364-e3372, 2020   103. Wiesmann M, Brockmann MA, Heringer S, Müller M, Reich A, Nikoubashman O : Active push deployment technique improves stent/vessel-wall interaction in endovascular treatment of acute stroke with stent retrievers. J Neurointerv Surg 9 : 253-256, 2017   104. Writing Group for the BASILAR Group; Zi W, Qiu Z, Wu D, Li F, Liu H, et al : Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol 77 : 561-573, 2020  105. Yoo AJ, Berkhemer OA, Fransen PSS, van den Berg LA, Beumer D, Lingsma HF, et al : Effect of baseline alberta stroke program early CT score on safety and efficacy of intra-arterial treatment: a subgroup analysis of a randomised phase 3 trial (MR CLEAN). Lancet Neurol 15 : 685-694, 2016   106. Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, González RG : MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke 40 : 2046-2054, 2009    107. Yoon W, Baek BH, Lee YY, Kim SK, Kim JT, Park MS : Association of pretreatment pontine infarction with extremely poor outcome after endovascular thrombectomy in acute basilar artery occlusion. J Neurointerv Surg 13 : 136-140, 2021   108. Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, et al : First pass effect: a new measure for stroke thrombectomy devices. Stroke 49 : 660-666, 2018   109. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al : Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 313 : 1240-1248, 2015   110. Zaidat OO, Liebeskind DS, Jadhav AP, Ortega-Gutierrez S, Nguyen TN, Haussen DC, et al : Impact of age and alberta stroke program early computed tomography score 0 to 5 on mechanical thrombectomy outcomes: analysis from the STRATIS registry. Stroke 52 : 2220-2228, 2021    111. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al : Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 44 : 2650-2663, 2013   
|
|



















