Byoun, Choi, Na, Kwon, and Nam: The Usefulness of Extradural Anterior Clinoidectomy for Lower-Lying Posterior Communicating Artery Aneurysms : A Cadaveric Study
Abstract
Objective
To confirm the usefulness of the extradural anterior clinoidectomy during the clipping of a lower riding posterior communicating artery (PCoA) aneurysm through cadaver dissection.
Methods
Anatomic measurements of 12 adult cadaveric heads (24 sides total) were performed to compare the microsurgical exposure of the PCoA and internal carotid artery (ICA) before and after clinoidectomy. A standard pterional craniotomy and transsylvian approach were performed in all cadavers. The distance from the ICA bifurcation to the origin of PCoA (D1), pre-anterior clinoidectomy distance from the ICA bifurcation to tentorium (D2), post-anterior clinoidectomy distance from the ICA bifurcation to tentorium (D3), pre-anterior clinoidectomy distance from the tentorium to the origin of PCoA (D4) and post-anterior clinoidectomy distance from the tentorium to the origin of PCoA (D5) and the distance of the ICA obtained after anterior clinoidectomy (D6) were measured. We measured the precise thickness of the blade for the Yasargil clip with a digital precision ruler to confirm the usefulness of the extradural anterior clinoidectomy.
Results
Twenty-four sites were dissected from 12 cadavers. The age of the cadavers was 79.83┬▒6.25 years. The number of males was the same as the females. The space from the proximal origin of the PCoA to the preclinoid-tentorium (D4) was 1.45┬▒1.08 mm (max, 4.01; min, 0.56). After the clinoidectomy, the space from the proximal origin of the PCoA to the postclinoid-tentorium (D5) was 3.612┬▒1.15 mm (max, 6.14; min, 1.83). The length (D6) of the exposed proximal ICA after the extradural clinoididectomy was 2.17┬▒1.04 mm on the lateral side and 2.16┬▒0.89 mm on the medial side. The thickness of the Yasargil clip blade used during the clipping surgery was 1.35 mm measured with a digital precision ruler.
Conclusion
The proximal length obtained by performing an external anterior clinoidectomy is about 2 mm, sufficient for proximal control during PCoA aneurysm surgery, considering the thickness of the aneurysm clips. In a subarachnoid hemorrhage, performing an extradural anterior clinoidectomy could prevent a devastating situation during PCoA aneurysm clipping.
Key Words: Aneurysm, posterior communicating artery ┬Ę Clip ┬Ę Cadaver ┬Ę Anterior clinoidectomy.
INTRODUCTION
As various new procedural techniques and endovascular devices have been developed during the endovascular era, the proportion of endovascular treatments (EVTs) in treating cerebral aneurysms has increased [ 5, 9, 10, 15, 18- 20]. In addition, as the use of antiplatelet agents and their long-term follow-up results have been reported, EVTs for cerebral aneurysms could have been implemented more reliably [ 1, 7, 8, 12, 16]. However, microsurgical clipping is still considered the primary treatment of cerebral aneurysms when EVTs cannot be performed, or microsurgical clipping has a relatively low risk. Proximal control greatly affects the success of microsurgical clipping surgery. When performing manipulations around the cerebral aneurysm, proximal control can reduce the risk of intraoperative aneurysm rupture by reducing the tension of the aneurysm wall. In case of an aneurysm rupture, we can obtain a clear surgical field and increase the accuracy of the aneurysm ligation through proximal control. In particular, for proximal control during microsurgical clipping of the posterior communicating artery (PCoA) aneurysm, the anatomical relationships between the anterior clinoid process (ACP) and other structures should be carefully considered. When proximal control is difficult due to the ACP, disaster can be prevented by securing the proximal portion from the posterior communicating segment of the internal carotid artery (ICA) through ACP removal or exposing the ipsilateral neck to trap the proximal ICA for proximal control.
A tailored intradural anterior clinoidectomy is a useful method to obtain a more proximal portion of the ICA to make proximal control precisely. However, it has some risks during the procedure in the case of subarachnoid hemorrhage (SAH) considering unclear surgical field, unstable condition of the aneurysm and so on [ 6, 26, 30]. The purpose of this study was to confirm the usefulness of the extradural anterior clinoidectomy during clipping of a lower riding PCoA aneurysm through cadaver dissection.
MATERIALS AND METHODS
Anatomic measurements of 12 adult cadaveric heads (24 sides total) were performed to compare the microsurgical exposure of the PCoA and ICA before and after clinoidectomy. Formalin-fixed human cadaveric heads were prepared with permission from the Department of Anatomy and the Institute for Applied Anatomy at The Catholic University of Korea, Seoul, Republic of Korea. All human tissues were secured in accordance with the tenets of the Declaration of Helsinki.
A standard pterional craniotomy and trans-Sylvian approach were performed in all cadavers. After sphenoid ridge flattening, proximal sylvian fissure splitting was performed to separate the frontal and temporal lobe. The distal portion of the ICA came into view. The dissection was continued to confirm the ICA bifurcation, the communicating segment of the ICA and the origin of the PCoA. The most proximal portion of the ICA contact with the tentorium protruded by the ACP was marked. The lengths were measured between the ICA bifurcation and the origin of the PCoA, the ICA bifurcation and the tentorium contacting the communicating segment of the ICA for both the lateral and medial aspects.
Then, extradural clinoidectomy was performed using highspeed drilling. After the extradural clinoidectomy, we measured the additional exposure of the proximal portion of the communicating segment of the ICA for both the lateral and medial aspects ( Fig. 1). The distance from the ICA bifurcation to the origin of the PCoA (D1), the pre-anterior clinoidectomy distance from the ICA bifurcation to the tentorium (D2), the post-anterior clinoidectomy distance from the ICA bifurcation to the tentorium (D3), the pre-anterior clinoidectomy distance from the tentorium to the origin of the PCoA (D4), and the post-anterior clinoidectomy distance from the tentorium to the origin of the PCoA (D5), and the distance of the ICA obtained after anterior clinoidectomy (D6) were measured ( Fig. 2). We measured the precise thickness of the blade for the Yasargil clip with a digital precision ruler to confirm the usefulness of the extradural anterior clinoidectomy ( Fig. 3).
Statistical analysis
The data are reported as the mean┬▒standard deviation. A pvalue of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (SPSS, Chicago, IL, USA).
RESULTS
Twenty-four sites were dissected from 12 cadavers. The age of the cadavers was 79.83┬▒6.25 years. The number of males was the same as the females.
The distance (D1) from the ICA bifurcation to the proximal origin of the PCoA on the lateral side of the ICA was 7.43┬▒1.65 mm. The distances (D2) from the ICA bifurcation to the tentorium measured before ACP removal were 8.88┬▒1.81 mm (lateral side) and 9.43┬▒1.96 mm (medial side). The distances (D3) from the ICA bifurcation to the tentorium measured again after the extradural clinoidectomy were 11.05┬▒1.67 mm (lateral side) and 11.60 ┬▒ 1.74 mm (medial side).
The space from the proximal origin of the PCoA to the preclinoid-tentorium (D4) was 1.45┬▒1.08 mm (max, 4.01; min, 0.56). After the clinoidectomy, the space from the proximal origin of the PCoA to the postclinoid-tentorium (D5) was 3.612┬▒1.15 mm (max, 6.14; min, 1.83). The length (D6) of the exposed proximal ICA after the extradural clinoididectomy was 2.17┬▒1.04 mm on the lateral side and 2.16┬▒0.89 mm on the medial side ( Table 1). The thickness of the Yasargil clip blade used during the clipping surgery was 1.35 mm, measured with a digital precision ruler ( Fig. 3).
DISCUSSION
Anatomical consideration
The supraclinoid portion of the ICA begins where the artery passes above the ACP to enter the subarachnoid space and terminate at the ICA bifurcation, and its average length is 14.8┬▒3.0 mm. We measured the distance from the ICA bifurcation to the point where the ICA view becomes obstructed by the ACP for both the lateral and medial ICA aspects. The mean values (D2) were 8.88┬▒1.81 mm (lateral side) and 9.43┬▒1.96 mm (medial side). Those values were smaller than the previously reported values. Evans et al. [ 3] reported that the mean value of the ICA length before removing the ACP was 10.5┬▒2.4 mm. Kim and Kang [ 13] also reported that the mean values of the supraclinoid ICA was 11.9┬▒2.3 mm. Racial differences and the shrunken arterial state during specimen preparation might cause differences in the measured length in previous reports.
Extradural anterior clinoidectomy for proximal control
After ACP removal, D3 was 11.05┬▒1.67 mm (lateral side) and 11.60┬▒1.74 mm (medial side). The additional lengths obtained through the ACP removal from both sides were 2.17┬▒1.04 mm (lateral side) and 2.16┬▒0.89 mm (medial side). The width of the clip blade was 1.35 mm for the Yasargil clip, mainly used for aneurysm clipping, and the L-clip was 1.20 mm.
The distance from the ICA bifurcation to the PCoA orifice was 7.43┬▒1.65 mm, and from the PCoA origin to the tentorium was 1.45┬▒1.08 mm. In 50% of the 24 sides of the 12 cadavers, there was not enough space to apply the temporary clip for proximal control. In these cases, the distance from the PCoA orifice to the tentorium was less than 1.35 mm.
Considering our results obtained through the cadaver study, sufficient space (about 2 mm) to carry out proximal control during clipping surgery could be obtained through only ACP removal.
The width of the clip blade currently used is 1.35 mm for the Yasargil clip and 1.20 mm for the L-clip.
If the distance from the PCoA orifice to the tentorium can be secured wider than the thickness of the clip blade, safe clipping for proximal control will be possible.
Considering our results obtained through cadaveric dissection, the length of the ICA exposed when only the ACP was removed was about 2 mm, which can sufficiently satisfy this condition.
Radiologic consideration for proximal control for PCoA aneurysm surgery
In the case of clipping for a PCoA aneurysm, radiological evaluations that required ACP removal have been reported in some studies. The distances from the ACP tip to the aneurysmal proximal neck on the sagittal plane (<4.0 to 5.6 mm), from the ACP line to the aneurysmal proximal neck (Ōēż0.5 to 2.0 mm), and an angle between the perpendicular line to the cranial base, and the axis of the communicating ICA (C7) with a greater angle between C7 and ophthalmic ICA (tortuosity of ICA) were the factors in which ACP removal was required [ 11, 21, 24, 25]. In addition to these reports, the distance of the ICA bifurcation-PCoA orifice measured in this study can be considered the other reference when deciding whether to remove the ACP. The distance from the ICA bifurcation to the tentorium (lateral side) (8.88┬▒1.81 mm) and the distance from the ICA bifurcation to the proximal margin of the PCoA (7.43┬▒1 mm) measured in this study were helpful to decide whether to perform ACP removal considering the width of the clips (1.20-1.35 mm).
The first consideration for proximal control during clipping surgery is the calcification of the vessel wall. Because, even if there is sufficient space for proximal control, severe calcification can cause the temporary clip to fail to apply or cause vascular injury [ 22]. If the significant vessel wall calcification is found on preoperative brain computed tomography angiography, it is safe to consider a neck exposure for proximal control. The second consideration is the relationship between the ACP and aneurysm. The shorter distances from the ACP tip to the aneurysmal proximal neck (<5.6 mm) and the ACP line to the aneurysmal proximal neck (<2.0 mm) are considered important factors for ACP removal during clipping surgery on previous studies [ 11, 21, 22, 24, 25]. According to our results, even if the effect of soft tissue surrounding the ACP is considered, the proximal distance secured by ACP removal in the actual surgical field of view (average 2 mm) is sufficient for proximal control. Therefore, the distance from the ACP tip to the origin of the PCoA also can be considered for ACP removal during clipping surgery.
Anterior clinoidectomy vs. anterior petroclinoid fold (APF) resection
According to a report from Helsinki, it was reported that an additional space of 2-3 mm could be secured with coagulation of the dura covering the ACP without ACP removal [ 30]. Other reports demonstrated that APF resection had several advantages, such as a simple and time-saving procedure during lower-lying PCoA aneurysm surgery, reducing the risk of damaging the surrounding neurovascular structure, avoiding cerebrospinal fluid leakage (CSF) leakage or pneumocephalus, and unnecessarily of exposing the proximal ICA to prepare proximal control [ 14, 17, 23]. However, the risk of intraoperative rebleeding by retraction or manipulation during the clipping of a ruptured aneurysm is higher than that of an unruptured aneurysm. Therefore, proximal control is more important in the case of SAH. The disadvantage is that it can be secured from the lateral side.
Coagulation of the dura covering the ACP and the APF resection had a disadvantage compared to ACP removal in proximal control. When ACP removal was performed, about 2 mm of space was secured on both the medial and lateral sides. Therefore, if the origin of the PCoA and the direction of the aneurysm are returned to the ventral side, it will help to apply the temporary and permanent clips safely.
Intradural vs. extradural anterior clinoidectomy
There are several advantages for intradural anterior clinoidectomy during clipping surgery, including a direct view and protection of adjacent neurovascular structures, usefulness with ACP variations such as an interclinoid osseous bridge and caroticoclinoid foramen. Additionally, we can reduce the surgical time and unnecessary procedures using a tailored anterior clinoidectomy (partial or subtotal removal of the ACP) [ 27, 29, 31]. However, an intradural anterior clinoidecotomy has the risk of a power drill-induced injury to adjacent structures and bone dust collection in the subarachnoid space [ 27, 29]. Especially, there is insufficient time to perform an anterior clinoidectomy, and it has an unclear surgical view in SAH. An external anterior clinoidectomy may be considered safe if ACP removal is necessary during clipping surgery for a ruptured PCoA aneurysm. A devastating situation can occur if a ruptured PCoA aneurysm rebleeds before the anterior clinoidectomy is completed. Possible complications can occur after an external dural clinoidectomy, including third cranial nerve injury, CSF, pneumocephalus and decreased vision [ 4, 27, 28, 30, 31]. However, an external anterior clinoidectomy is safer than an intradural anterior clinoidectomy in terms of dural protection for the adjacent neurovascular structures, no bone dust collection in the intradural space, and a relatively clear surgical field during the procedure in a SAH. There are several precautions to prevent complications while performing an external anterior clinoidectomy. First, the ACP should be centrally shelled or hollowed out using a diamond drill so that the walls can be easily fractured and circumferentially dissected free of the surrounding dural folds. During drilling, continuous irrigation should be performed. Sometimes, it is better to use ultrasonic bone aspirators, having no rotating components, which are presented as a more stable and safer method [ 2]. Second, en bloc removal of the ACP should be avoided as much as possible, as it would likely require additional extradural manipulation. The risk imposed by this action involves damage to the oculomotor nerve, which runs close to the lateral undersurface of the ACP [ 31]. Third, bleeding from the anterior part of the roof of the cavernous sinus may occur, but it is easy to control using oxycellulose with cotton sponge compression. However, it is easy to damage the oculomotor nerve by tight packing in this area [ 28].
Limitations of the study
There are some limitations in our study. First, this study used an experimental design with cadaver dissection. Cadaveric tissues lack elasticity and have a shorter length than living tissues, which may cause errors in actual measurements. Because this study focused on the bone (ACP), there would be fewer measurement errors. Second, there are racial differences in structures, including the size and shape of the head, length of the intracranial vessels, and size of the ACP. Our results do not reflect a non-Asian head anatomy. However, this study can be used as one of the references for surgery of lower-lying PCoA aneurysms in Asians.
CONCLUSION
The proximal length obtained by performing an external anterior clinoidectomy is about 2 mm, sufficient for proximal control during PCoA aneurysm surgery, considering the thickness of the aneurysm clips. If sufficient space for proximal control is not ensured through radiological examination before the PCoA aneurysm clipping surgery, surgery can be performed through APF resection or anterior clinoidectomy. In SAH, performing an extradural anterior clinoidectomy might prevent a devastating situation during lower-lying PCoA aneurysm clipping.
Fig.┬Ā1.
Microscopic view of (A) intradural surgical field before extradural anterior clinoidectomy, (B) extradural surgical field after extradural anterior clinoidectomy, and (C) intradural surgical field after extradural anterior clinoidectomy after left pterional approach. The purple line on the internal carotid artery is the tentorial margin before the extradural anterior clionoidectomy. 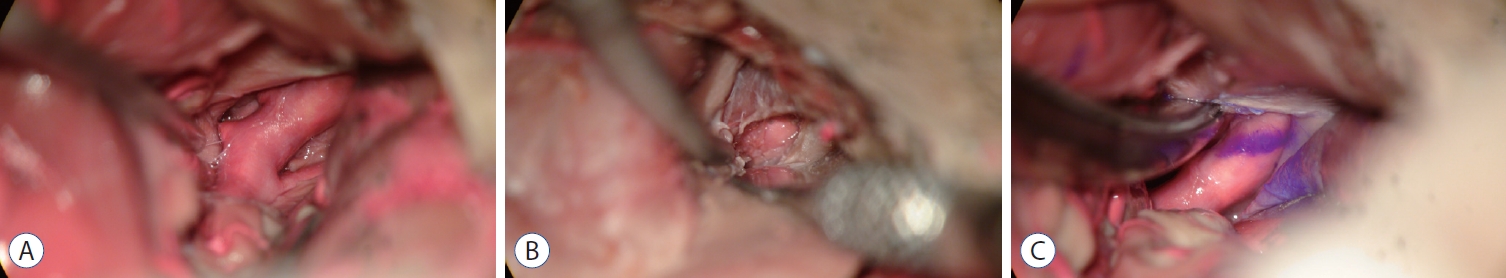
Fig.┬Ā2.
Comparative illustration of the intradural surgical field (A) before and (B) after the extradural anterior clinoidectomy. The blue dotted line is the margin of the anterior clinoid process (ACP) before the extradural anterior clinoidectomy. D2L : lateral side of distance 2, Pcom : posterior communicating artery, D1 : distance 1, D4 : distance 4, M : middle cerebral artery, A1 : A1 portion of the anterior cerebral artery, D2M : medial side of distance 2, D3L : lateral side of distance 3, D5 : distance 5, D3M : medial side of distance 3. 
Fig.┬Ā3.
Digital precision ruler to measure the thickness of the aneurysm clip blade. 
Table┬Ā1.
The basic characteristic of the 24 side of the 12 cadavers
|
Medial side |
Lateral side |
p-value |
|
D1 (ICAB-Pc) |
|
7.43┬▒1.65 |
|
|
D2 (ICAB-Te1) |
9.43┬▒1.96 |
8.88┬▒1.81 |
0.314 |
|
D3 (ICAB-Te2) |
11.60┬▒1.74 |
11.05┬▒1.67 |
0.268 |
|
D4 (Te1-Pc) |
|
1.45┬▒1.08 |
|
|
D5 (Te2-Pc) |
|
3.612┬▒1.15 |
|
|
D6 (D3-D2) |
2.16┬▒0.89 |
2.17┬▒1.04 |
0.991 |
References
1. Almekhlafi MA, Al Sultan AS, Kuczynski AM, Brinjikji W, Menon BK, Hill MD, et al : Antiplatelet therapy for prevention of thromboembolic complications in coiling-only procedures for unruptured brain aneurysms. J Neurointerv Surg 12 : 298-302, 2020   2. Chang HS, Joko M, Song JS, Ito K, Inoue T, Nakagawa H : Ultrasonic bone curettage for optic canal unroofing and anterior clinoidectomy. Technical note. J Neurosurg 104 : 621-624, 2006  3. Evans JJ, Hwang YS, Lee JH : Pre- versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery, and opticocarotid triangle: a cadaveric morphometric study. Neurosurgery 46 : 1018-1021; discussion 1021-1023, 2000   4. Froelich SC, Aziz KM, Levine NB, Theodosopoulos PV, van Loveren HR, Keller JT : Refinement of the extradural anterior clinoidectomy: surgical anatomy of the orbitotemporal periosteal fold. Neurosurgery 61 : 179-185; discussion 185-186, 2007  5. Golnari P, Nazari P, Garcia RM, Weiss H, Shaibani A, Hurley MC, et al : Volumes, outcomes, and complications after surgical versus endovascular treatment of aneurysms in the United States (1993-2015): continued evolution versus steady-state after more than 2 decades of practice. J Neurosurg 134 : 848-861, 2020   6. Gon├¦alves Pacheco Junior M, de Melo Junior JO, Andr├® Acioly M, Mansilla Cabrera Rodrigues R, Lima Pess├┤a B, Fernandes RA, et al : Tailored anterior clinoidectomy: beyond the intradural and extradural concepts. Cureus 13 : e14874, 2021   7. Hwang G, Huh W, Lee JS, Villavicencio JB, Villamor RB Jr, Ahn SY, et al : Standard vs modified antiplatelet preparation for preventing thromboembolic events in patients with high on-treatment platelet reactivity undergoing coil embolization for an unruptured intracranial aneurysm: a randomized clinical trial. JAMA Neurol 72 : 764-772, 2015   8. Hwang G, Jung C, Park SQ, Kang HS, Lee SH, Oh CW, et al : Thromboembolic complications of elective coil embolization of unruptured aneurysms: the effect of oral antiplatelet preparation on periprocedural thromboembolic complication. Neurosurgery 67 : 743-748; discussion 748, 2010  10. Jiang Z, Chen Y, Zeng C, Feng J, Wan Y, Zhang X : Neurosurgical clipping versus endovascular coiling for patients with intracranial aneurysms: a systematic review and meta-analysis. World Neurosurg 138 : e191-e222, 2020   11. Kamide T, Burkhardt JK, Tabani H, Safaee MM, Lawton MT : Preoperative prediction of the necessity for anterior clinoidectomy during microsurgical clipping of ruptured posterior communicating artery aneurysms. World Neurosurg 109 : e493-e501, 2018   12. Kim CH, Hwang G, Kwon OK, Ban SP, Chinh ND, Tjahjadi M, et al : P2Y12 reaction units threshold for implementing modified antiplatelet preparation in coil embolization of unruptured aneurysms: a prospective validation study. Radiology 282 : 542-551, 2017   13. Kim DW, Kang SD : Association between internal carotid artery morphometry and posterior communicating artery aneurysm. Yonsei Med J 48 : 634-638, 2007    14. Kim JH, Kim JM, Cheong JH, Bak KH, Kim CH : Simple anterior petroclinoid fold resection in the treatment of low-lying internal carotidposterior communicating artery aneurysms. Surg Neurol 72 : 142-145, 2009   16. Li W, Zhu W, Wang A, Zhang G, Zhang Y, Wang K, et al : Effect of adjusted antiplatelet therapy on preventing ischemic events after stenting for intracranial aneurysms. Stroke 52 : 3815-3825, 2021   17. Matano F, Murai Y, Mizunari T, Yamaguchi M, Yamada T, Baba E, et al : Incision of the anterior petroclinoidal fold during clipping for securing the proximal space of an internal carotid artery-posterior communicating artery aneurysm: a technical note. Neurosurg Rev 42 : 777-781, 2019    18. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al : International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360 : 1267-1274, 2002   19. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS : The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 385 : 691-697, 2015    20. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al : International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366 : 809-817, 2005   21. Nagasawa S, Kikuchi H, Kim NG, Yonekawa Y : Analysis of internal carotid-posterior communicating artery aneurysms with difficulty in clipping: with special reference to radiometry. No Shinkei Geka 16 : 959-964, 1988  22. Niibo T, Takizawa K, Sakurai J, Takebayashi S, Koizumi H, Kobayashi T, et al : Prediction of the difficulty of proximal vascular control using 3DCTA for the surgical clipping of internal carotid artery-posterior communicating artery aneurysms. J Neurosurg 134 : 1165-1172, 2020   23. Nossek E, Setton A, Dehdashti AR, Chalif DJ : Anterior petroclinoid fold fenestration: an adjunct to clipping of postero-laterally projecting posterior communicating aneurysms. Neurosurg Rev 37 : 637-641, 2014    24. Ochiai C, Wakai S, Inou S, Nagai M : Preoperative angiographical prediction of the necessity to removal of the anterior clinoid process in internal carotid-posterior communicating artery aneurysm surgery. Acta Neurochir (Wien) 99 : 117-121, 1989    25. Park SK, Shin YS, Lim YC, Chung J : Preoperative predictive value of the necessity for anterior clinoidectomy in posterior communicating artery aneurysm clipping. Neurosurgery 65 : 281-285; discussion 285-286, 2009    26. Romani R, Elsharkawy A, Laakso A, Kangasniemi M, Hernesniemi J : Tailored anterior clinoidectomy through the lateral supraorbital approach: experience with 82 consecutive patients. World Neurosurg 77 : 512-517, 2012   27. Salgado L├│pez L, Mu├▒oz Hern├Īndez F, Asencio Cort├®s C, Tresserras Rib├│ P, ├ülvarez Holzapfel MJ, Molet Teixid├│ J : Extradural anterior clinoidectomy in the management of parasellar meningiomas: analysis of 13 years of experience and literature review. Neurocirugia (Astur : Engl Ed) 29 : 225-232, 2018   28. Son HE, Park MS, Kim SM, Jung SS, Park KS, Chung SY : The avoidance of microsurgical complications in the extradural anterior clinoidectomy to paraclinoid aneurysms. J Korean Neurosurg Soc 48 : 199-206, 2010    29. Tayebi Meybodi A, Lawton MT, Yousef S, Guo X, Gonz├Īlez S├Īnchez JJ, Tabani H, et al : Anterior clinoidectomy using an extradural and intradural 2-step hybrid technique. J Neurosurg 130 : 238-247, 2018   30. Thiarawat P, Jahromi BR, Kozyrev DA, Intarakhao P, Teo MK, ChoqueVelasquez J, et al : Microneurosurgical management of posterior communicating artery aneurysm: a contemporary series from Helsinki. World Neurosurg 101 : 379-388, 2017   31. Yonekawa Y, Ogata N, Imhof HG, Olivecrona M, Strommer K, Kwak TE, et al : Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg 87 : 636-642, 1997 
|
|



















