Eun and Park: Early Detection of hyperemia with Magnetic Resonance Fluid Attenuation Inversion Recovery Imaging after Superficial Temporal Artery to Middle Cerebral Artery Anastomosis
Abstract
Objective
Cerebral hyperperfusion syndrome (CHS) manifests as a collection of symptoms brought on by heightened focal cerebral blood flow (CBF), afflicting nearly 30% of patients who have undergone superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis. The aim of this study was to investigate whether the amalgamation of magnetic resonance imaging (MRI) fluid-attenuated inversion recovery (FLAIR) and apparent diffusion coefficient (ADC) imaging via MRI can discern cerebral hyperemia after STA-MCA anastomosis surgery.
Methods
A retrospective study was performed of patients who underwent STA-MCA anastomosis due to Moyamoya disease or atherosclerotic steno-occlusive disease. A protocol aimed at preventing CHS was instituted, leveraging the use of MRI FLAIR. Patients underwent MRI diffusion with FLAIR imaging 24 hours after STA-MCA anastomosis. A high signal on FLAIR images signified the presence of hyperemia at the bypass site, triggering a protocol of hyperemia care. All patients underwent hemodynamic evaluations, including perfusion MRI, single-photon emission computed tomography (SPECT), and digital subtraction angiography, both before and after the surgery. If a high signal intensity is observed on MRI FLAIR within 24 hours of the surgery, a repeat MRI is performed to confirm the presence of hyperemia. Patients with confirmed hyperemia are managed according to a protocol aimed at preventing further progression.
Results
Out of a total of 162 patients, 24 individuals (comprising 16 women and 8 men) exhibited hyperemia on their MRI FLAIR scans following the procedure. SPECT was conducted on 23 patients, and 11 of them yielded positive results. All 24 patients underwent perfusion MRI, but nine of them showed no significant findings. Among the patients, 10 displayed elevations in both CBF and cerebral blood volume (CBV), three only showed elevation in CBF, and two only showed elevation in CBV. Follow-up MRI FLAIR scans conducted 6 months later on these patients revealed complete normalization of the previously observed high signal intensity, with no evidence of ischemic injury.
Conclusion
The study determined that the use of MRI FLAIR and ADC mapping is a competent means of early detection of hyperemia after STA-MCA anastomosis surgery. The protocol established can be adopted by other neurosurgical institutions to enhance patient outcomes and mitigate the hazard of permanent cerebral injury caused by cerebral hyperemia.
Key Words: Cerebral revascularization · Cerebrovascular circulation · Hyperemia.
INTRODUCTION
Cerebral hyperperfusion syndrome (CHS) is a condition characterized by symptoms such as headache, aphasia, hemiparesis, and seizure that occurs after revascularization surgery. This is due to an increase in focal cerebral blood flow (CBF), which can lead to vasogenic edema and further neurological deterioration [ 1, 3, 6, 20, 27]. The incidence of symptomatic CHS after superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis is around 20-30% [ 5, 12, 18]. Based on this, it is likely there are more patients suffering from cerebral hyperemia after anastomosis, including those who are asymptomatic. Therefore, a routine protocol for early and accurate diagnosis, along with proper management of cerebral hyperemia after STA-MCA anastomosis, is necessary to prevent permanent neurologic sequelae. The diagnosis of CHS is made using symptoms and single-photon emission computed tomography (SPECT), perfusion computed tomography (CT), or magnetic resonance imaging (MRI). SPECT is considered the standard modality [ 3, 11, 26, 27]; however, it may not be available in emergency situations [ 2]. If SPECT couldn’t be performed at the exact moment of a hyperemic state, hypoperfusion could paradoxically be detected [ 11, 14, 23]. We aimed to establish a quicker diagnostic process for detecting cerebral hyperemia immediately. The objective of this study was to assess the efficacy of utilizing MRI fluid-attenuated inversion recovery (FLAIR) in conjunction with apparent diffusion coefficient (ADC) imaging as an early diagnostic tool for the detection of cerebral hyperemia after STA-MCA anastomosis and to propose a management protocol based on MRI results.
MATERIALS AND METHODS
This research was approved by the Institutional Review Board of the Catholic University of Korea (IRB No. HC21RISI0092) and was conducted in accordance with the Declaration of Helsinki.
A retrospective study was conducted on patients who had undergone bypass surgery among those with perfusion defect due to Moyamoya disease (MMD) or intracranial atherosclerotic steno-occlusive disease, where the Tmax6 volume on the time to peak map was greater than 60 mL. The study involved investigating medical records and images. Patients who did not have as follow-up of at least 6 months after surgery or lacked medical records were excluded.
From October 2016 to November 2021, a protocol for preventing CHS incorporating MRI FLAIR was introduced at our institution. Before surgery, digital subtraction angiography, SPECT, and MRI exams, which included T1-/T2-weighted imaging, enhanced T1-weighted imaging, FLAIR imaging, diffusion-weighted imaging, time of flight (TOF), magnetic resonance angiography (MRA), were performed. The surgical method for each patient was determined based on preoperative digital subtraction angiography, SPECT, and perfusion MRI. The donor STA was anastomosed to cortical MCA (M4) in an end-to-side manner with 10-0 nylon. If the post-anastomosis flow was confirmed to be <50% of the cut flow, based on the microvascular ultrasonic flowmeter (Charbel microflow probe; Transonics Systems, Inc., Ithaca, NY, USA), additional anastomosis with the remaining donor vessel was performed. Bypass patency in every anastomosis case was confirmed with intraoperative micro-Doppler measurement (Nicolet Biomedical, Madison, WI, USA) and indocyanine green angiography.
Protocol for preventing CHS after STA-MCA anastomosis
Following the STA-MCA anastomosis procedure, CT angiography was performed 6 hours postoperatively, and an MRI diffusion with FLAIR imaging was taken 24 hours postoperatively. If a high signal on the FLAIR image, indicative of hyperemia at the bypass site, was present, a pre-determined protocol was implemented, encompassing absolute bed rest, systolic blood pressure regulation to <120 mmHg, and administration of anticonvulsant and steroids, all with the aim of averting neurologic impairment. If there were no significant findings on the FLAIR image, patients were permitted to ambulate and subjected to routine postoperative care. On postoperative day 7, they underwent perfusion MRI and SPECT prior to discharge. The reason for performing images studies is to confirm the improvement of preoperative perfusion defects. If any neurologic symptoms arose during hospitalization, MRI diffusion with FLAIR and perfusion MRI was performed, and, in the event of hyperemia being detected, the protocol of absolute bed rest, blood pressure control, anticonvulsant administration, and steroid treatment was implemented for symptom mitigation. Upon alleviation of neurologic symptoms, the patient was then discharged ( Fig. 1). The diagnosis of cerebral vasogenic edema was established through the evaluation of certain radiologic indications by two neuroradiologists with over 10 years of experience in the field, which included : 1) the emergence of new high signal intensity as indicated by FLAIR and ADC mapping of the hemisphere that underwent surgical treatment; 2) the preservation of anastomosis vessel patency; and 3) the lack of postoperative complications such as ischemic lesions, infections, or subdural hemorrhage (as depicted in Fig. 2). In cases where two neuroradiologists agree with the three findings above, the final diagnosis is determined to be as cerebral vasogenic edema.
Statistical method
The statistical analysis was performed using R version 4.2.2 (IR Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were calculated using median and interquartile range values. Fisher’s exact test was used to analyze the differences between symptomatic and asymptomatic patients. p-values were two-sided, with a confidence level of 0.95.
RESULTS
Of the 162 patients who underwent STA-MCA anastomosis, 24 were found to exhibit hyperemia on MRI FLAIR images. The subset of patients with hyperemia had a median age of 59.5 years, with a sex distribution of 16 female patients and eight male patients. Many of the operations, 16 in total, were performed on the left side, while the remaining eight were performed on the right side. A double-barrel procedure using two STA branches was performed on 10 patients, with three of these cases also featuring combined encephalo-duro-syangiosis (EDAS). The remaining 14 patients underwent a single-barrel procedure using one STA branch, and seven of these patients also underwent combined EDAS. The median cut flow of STAs of patients was 49.00 mL/min (23.50-54.00), and the anastomosis flow after surgery was 21.00 mL/min (16.75- 33.25). Symptomatic patients had a median onset time of 1.5 days (1-3). Six patients reported verbal abnormalities, such as dysarthria or aphasia. Three patients reported transient hemiparesis, and two patients were diagnosed with CHS after seizure-like movements. One patient showed facial palsy, and another reported severe dizziness. In the asymptomatic group, there were 10 female patients and one male patient, while, in the symptomatic group, there were six female patients and seven male patients ( p=0.033). Five asymptomatic individuals underwent left-side procedures and six underwent right-side procedures, while 11 symptomatic patients underwent left-side procedures and two underwent right-side procedures ( p=0.082). Seven patients in the asymptomatic group underwent double-barrel operations, including three treated with EDAS, and four asymptomatic cases underwent single-barrel operations, including one treated with EDAS. Among the symptomatic patients, three underwent double-barrel operations, while 10 underwent single-barrel bypass operations, including six treated with EDAS ( p=0.086) ( Table 1). SPECT was performed on 23 patients, with 11 presenting positive findings. Perfusion MRI was completed on all 24 patients, but nine showed no specific findings. Ten patients showed an increase in both CBF and CBV, while others showed an elevation of only CBF or CBV. However, all patients who underwent follow-up FLAIR MRI at 6 months showed full recovery of the hyperemia observed on the initial MRI FLAIR image. SPECT imaging was performed on 10 asymptomatic patients and all symptomatic patients, with five asymptomatic patients and six symptomatic patients demonstrating positive results. All patients underwent perfusion MRI, with negative findings identified in three asymptomatic patients and six symptomatic patients, respectively (as displayed in Table 2). The study compared the effectiveness of different diagnostic tools, including perfusion MRI, SPECT, and FLAIR. The results showed that FLAIR had 100% sensitivity in diagnosing cerebral hyperemia, while SPECT showed 47.83% sensitivity and perfusion MRI showed 59.09% sensitivity ( Table 3).
DISCUSSION
Carotid endarterectomy (CEA) is where CHS was first recognized as a significant postoperative complication. Standard protocols are employed post-surgery to avoid CHS, including keeping systolic blood pressure to <150 mmHg. Unlike CEA, STA-MCA anastomosis is a low-flow bypass that results in reduced CBF. In 1998, Uno et al. [ 24] reported CHS results among MMD patients who underwent STA-MCA anastomosis, leading to numerous studies on CHS after such procedures. A meta-analysis of 26 cohort studies conducted by Yu et al. [ 26] recorded a CHS incidence of 16.5% (range, 11.3-22.3%) after STA-MCA anastomosis for MMD, which is higher than rates of other postoperative complications such as infarction, occlusion, hypoperfusion, infection, and other rare occurrences. Other studies have recorded a symptomatic CHS incidence after low-flow bypass in the range of 20-30% [ 5, 12, 18]. Although CHS has a high incidence rate, it usually proves temporary, and complete neurologic recovery can be achieved within 14 postoperative days with proper management [ 6, 9, 11, 23, 26]. On the other hand, if CHS is not detected and treated promptly, brain parenchymal damage may occur, leading to permanent neurologic consequences [ 1, 6, 20, 26]. As previous studies mostly enrolled symptomatic patients, the actual hyperemia incidence after STA-MCA anastomosis is likely greater than what is reported. The origin of CHS remains enigmatic, with multiple hypotheses positing contributing elements such as disrupted cerebral autoregulation, augmented CBF post-revascularization, and hemodynamic stress. Chronic stenotic occlusive disease and MMD may give rise to dilated and hypertrophied vessels, which exhaust cerebrovascular reactivity in turn and interfere with cerebral autoregulation [ 3, 8, 13, 25, 27]. The liberation of oxygen-free radicals during reperfusion may also trigger vasodilation and increased vascular permeability [ 10, 15, 17, 25, 27]. Postoperative hypertension can worsen CHS [ 1]. In the normal population, when blood pressure rises, cerebral blood vessels constrict, reducing CBF to preserve the brain. However, in those with chronic ischemia, cerebral blood vessels, whose autoregulation is disrupted, do not exhibit a normal response to reduce the blood flow even when the blood pressure rises. In addition, since CBF is increased by bypass surgery, the risk of brain damage increases due to increased CBF. As a result, hypertension can lead to an increase in focal CBF with vasogenic edema [ 3, 6]. Vasogenic edema occurs when increased pressure in the blood vessels leads to the accumulation of fluid in the brain tissue, causing the tissue to swell. In such cases, medical attention should be sought as it can be a sign of severe brain injury [ 3, 6]. STA-MCA anastomosis is a low-flow bypass procedure with a lower risk of hyperemia compared to CEA. In addition, if the blood pressure is too low, there is a risk of additional ischemic brain damage after surgery. Therefore, it is not recommended to lower the blood pressure in a uniform manner of a patients who has undergone surgery, even if it is slightly high. However, when hyperemia occurs, blood pressure must be lowered as soon as possible to reduce brain damage. Therefore, we sought to establish an early detection method for CHS in STA-MCA anastomosis patients. In our hospital before our protocol was established, there were six patients with CHS after STA-MCA anastomosis. All patients showed vasogenic edema in the subcortical white matter, characterized by high signal intensity on both the FLAIR and ADC maps. Five of fix patients underwent SPECT, but all showed negative results. One patient showed elevated levels of both CBV and CBF on perfusion MRI, one patient showed only elevation of CBF, and two patients showed no hyperemia at all. Neurologic symptoms occurred at a median of 5 days (1-10) postoperatively. Patients reported dysarthria, seizure, and side weakness. One of the severe cases, there was a patient admitted with a modified Rankin scale of 1, developed CHS after the anastomosis and discharged with a modified Rankin scale of 3 after more than 3 months of hospitalization. The operation was performed on the left side in three patients and on the right side in three patients. When comparing symptomatic patients to asymptomatic patients in our study, there was a borderline significant difference in the number of patients with operations on the left side. Because the left hemisphere is usually the dominant hemisphere for most patients [ 7, 22], the operator should inform patients and be cautious about the possibility of CHS before planning an STA-MCA anastomosis on the left side. CHS has been defined in several studies as an increase of CBF of >100% compared to the preoperative baseline CBF in CEA and carotid artery stenting [ 3, 8, 16, 20, 25]. However, there is currently no consensus on the exact definition of CHS following STA-MCA anastomosis. Many studies use different imaging tools to detect increases in CBF for CHS diagnosis. SPECT is widely used as a standard modality for evaluating CBF and for differentiating CHS from ischemia [ 3, 11, 26, 27]. However, SPECT has limitations as it is time-consuming, requires a radioactive tracer, and cannot be used in many hospitals for emergency diagnosis. Additionally, cerebral hyperemia can be transient and intermittent, so imaging to evaluate CBF must be performed at the exact time of hyperemia or serially to compare states [ 11, 14, 23]. The results of SPECT in several studies have varied based on when the imaging was performed serially to evaluate CBF [ 23]. In this study, both SPECT and perfusion MRI showed variable results. Also, one report described CHS diagnosis with RAPID perfusion CT. RAPID perfusion CT was used to diagnose CHS by showing a relationship between perfusion CT and CHS on postoperative day 4 [ 19]. Other imaging modalities that do not directly measure CBF for CHS diagnosis have been reported, such as TOF-MRA suggested by Sato et al. [ 21]. The change ratio of signal intensity on TOF-MRA can be useful for screening CHS because the signal intensity of the anastomosis vessel is increased in CHS patients. Many studies have emphasized the importance of early detection and management of CHS to prevent permanent parenchymal damage [ 1, 3, 6, 20, 26]. In this study, the focus is on identifying the most rapid and convenient method for diagnosing vasogenic edema caused by hyperemia, which is MRI FLAIR. When combined with diffusion-weighted imaging, it can differentiate cerebral edema caused by ischemia. Our study found that all patients diagnosed with CHS showed high signal intensity in the subcortical white matter on both FLAIR and ADC maps, and every patient except one with dysarthria recovered fully with appropriate treatment. The study also compared the effectiveness of different diagnostic tools. In the patient example in Fig. 3, significant hyperemia was observed in the left frontal lobe, but there were unclear reductions in CBV and CBF detected by perfusion MRI. Additionally, SPECT was unable to detect hyperemia. On the other hand, in Fig. 4, another patient showed an elevation of CBF, which could be detected by perfusion MRI. Overall, the study highlights the effectiveness of MRI FLAIR in diagnosing bypass-related hyperemia and suggests that it is a useful diagnostic tool for CHS. One of important points of this analysis is that patients who underwent follow-up MRI FLAIR 6 months later showed no high signal intensity at all and also no ischemic injury. It means that high signal intensity on MRI FLAIR after STA-MCA anastomosis can be interpreted as reversible change of cerebral edema. Many studies have shown that careful monitoring and lowering of blood pressure are critical for managing CHS [ 3, 4, 25, 26]. However, there is a lack of consensus about the target of blood pressure and the duration of anti-hypertensive treatment. In this study, systolic blood pressure was carefully lowered to <120 mmHg using intravenous labetalol or nicardipine during the symptomatic period. Anti-epileptic drugs, corticosteroids, and mannitol were used in selected patients. Edaravone, a free radical scavenger that has been reported to be effective against CHS in some studies [ 15] was not used in this study due to a lack of availability.
Limitation
Because this analysis is based on the selection of patients according to a specific protocol, it has limitations in terms of statistical proof. Therefore, further research on a larger population is needed.
CONCLUSION
Cerebral hyperemia refers to an increase in blood flow to the brain. MRI FLAIR imaging can be used to diagnose cerebral hyperemia immediately, regardless of timing, and, when performed with ADC mapping, it can differentiate cerebral hyperemia from ischemia. MRI can detect even minimal vasogenic edema areas, allowing for early and accurate diagnosis of cerebral hyperemia. The use of MRI FLAIR combined with ADC mapping is a convenient and effective way to diagnose focal hyperemia after STA-MCA anastomosis. This has allowed for early detection and management of CHS, reducing the risk of permanent damage to the brain.
Fig. 1.
Protocol. STA : superficial temporal artery, MCA : middle cerebral artery, CT : computed tomography, MRI : magnetic resonance imaging, FLAIR : fluid-attenuated inversion recovery, POD : post operative day. 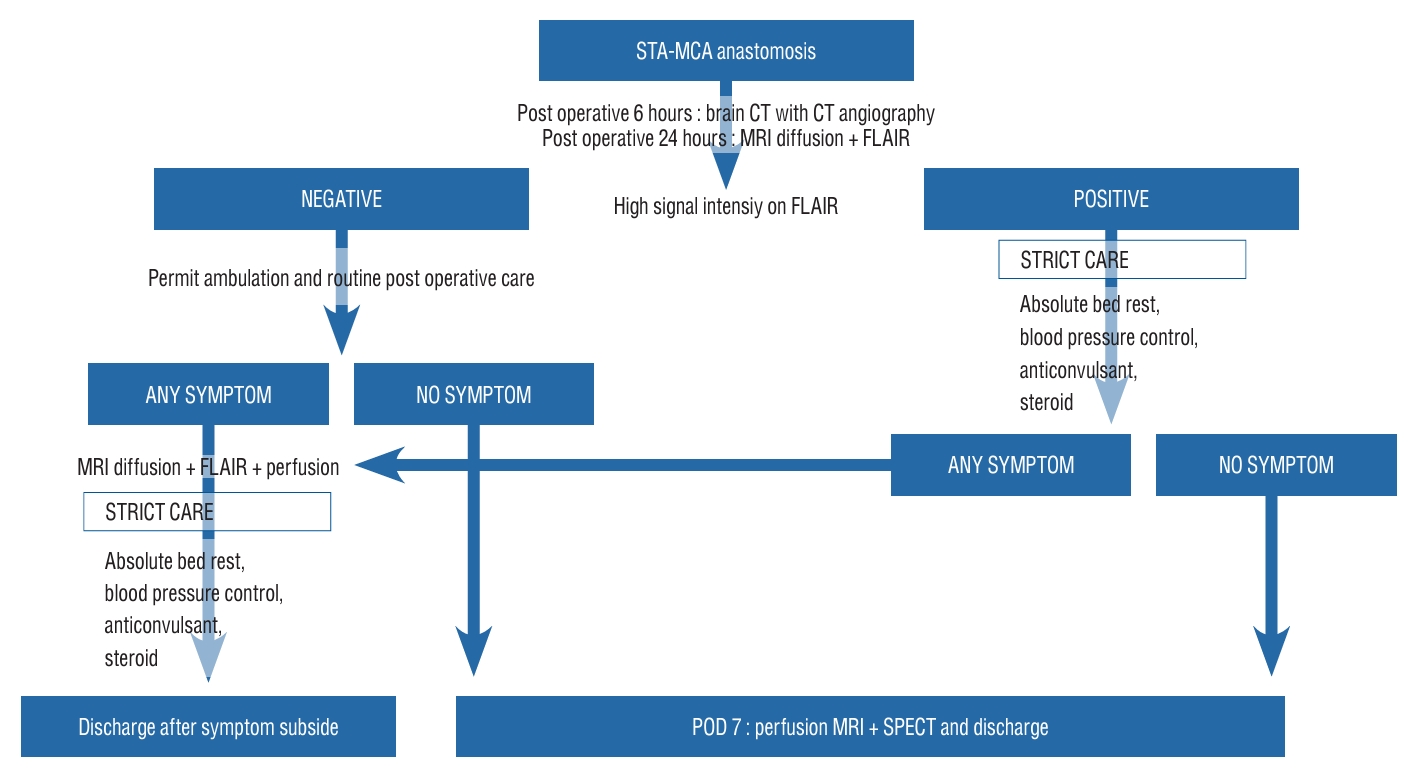
Fig. 2.
Postoperative MRI FLAIR (A) and ADC (B) images. At postoperative day 1, a high signal intensity area was observed on the right frontal lobe (arrow in A) indicating vasogenic edema on FLAIR. A high signal intensity area was observed on the ADC image (arrow in B) in the same area indicating interstitial edema not cerebral infarction. MRI : magnetic resonance imaging, FLAIR : fluid-attenuated inversion recovery, ADC : apparent diffusion coefficient. 
Fig. 3.
A case illustrations of postoperative MRI FLAIR, perfusion MRI, and SPECT image. A : A high signal intensity area was observed on the left frontal lobe (arrows) indicating vasogenic edema on FLAIR. B : A perfusion MRI of the same patients revealed a slight decrease in cerebral blood volume and postoperative SPECT showed a decrease in blood flow at the bypass site. C : A follow up MRI taken 6 months later showed complete recovery of hyperemia in FLAIR image (arrow), indicating that the vasogenic edema had resolved. FLAIR : fluid-attenuated inversion recovery, ADC : apparent diffusion coefficient, DWI : diffusion weighted imaging, CBF : cerebral blood flow, TTP : time-to-peak, SPECT : single-photon emission computed tomography, MRI : magnetic resonance imaging. 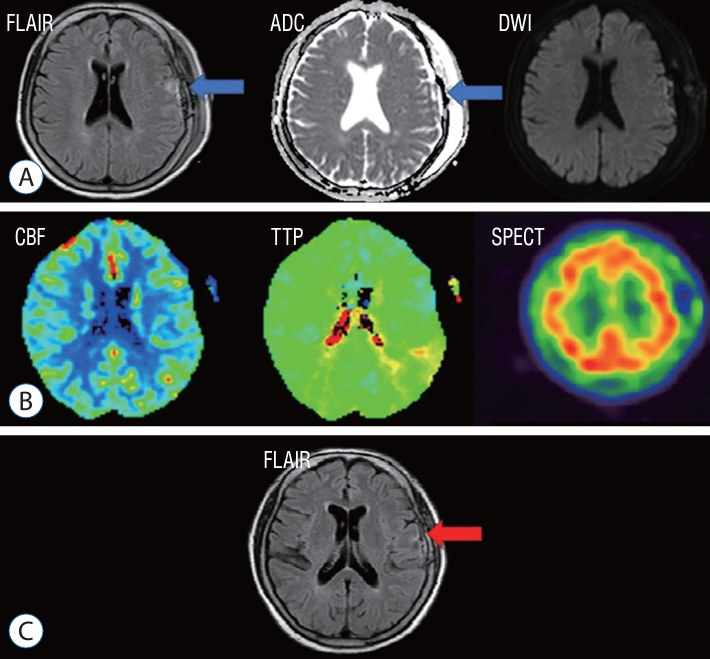
Fig. 4.
A case illustration of MRI FLAIR, SPECT, and perfusion MRI. A : An MRI FLAIR image showed a high signal intensity area in the right frontal lobe (arrows), indicating vasogenic edema. The same perfusion MRI showed increased cerebral blood flow at the site of the operation. B : SPECT showed increased flow on operation site (arrows). C : The postoperative MRI FLAIR taken 2 months after surgery showed that the previously observed lesion had normalized (arrow). FLAIR : fluid-attenuated inversion recovery, CBF : cerebral blood flow, TTP : time-to-peak, SPECT : single-photon emission computed tomography, f/u : follow-up, MRI : magnetic resonance imaging. 
Table 1.
Baseline characteristics of patients with hyperemia
|
All (n=24) |
Asymptomatic patients (n=11) |
Symptomatic patients (n=13) |
p-value |
|
Age (years) |
59.5 (53.75-64) |
60.00 (51.50-64.00) |
59.00 (57.00-64.00) |
0.913 |
|
Sex |
|
|
|
0.033 |
|
Male |
8 |
1 (9.09) |
7 (53.85) |
|
|
Female |
16 |
10 (90.91) |
6 (46.15) |
|
|
Diagnosis |
|
|
|
1.000 |
|
ICAD |
13 |
6 (54.55) |
7 (53.85) |
|
|
MMD |
11 |
5 (45.45) |
6 (46.15) |
|
|
Operation side |
|
|
|
0.082 |
|
Left |
16 |
5 (45.45) |
11 (84.62) |
|
|
Right |
8 |
6 (54.55) |
2 (15.38) |
|
|
Operation method |
|
|
|
0.086 |
|
Double-barrel |
10 |
7 |
3 |
|
|
Including EDAS |
3 |
3 |
0 |
|
|
Single-barrel |
14 |
4 |
10 |
|
|
Including EDAS |
7 |
1 |
6 |
|
|
Cut flow |
49.00 (23.50-54.00) |
50.00 (33.50-55.50) |
40.00 (19.00-50.00) |
0.851 |
|
After anastomosis flow |
21.00 (16.75-33.25) |
20.00 (15.50-28.00) |
21.00 (16.75-38.00) |
0.718 |
Table 2.
Comparison of hyperemia patients according to the presence of symptoms
|
All |
Asymptomatic patients |
Symptomatic patients |
|
FLAIR+ADC |
|
|
|
|
Yes |
24 (100.00) |
11 (100.00) |
13 (100.00) |
|
No |
0 (0.00) |
0 (0.00) |
0 (0.00) |
|
SPECT |
|
|
|
|
Yes |
11 |
5 (50.00) |
6 (46.15) |
|
No |
12 |
5 (50.00) |
7 (53.85) |
|
Not performed |
1 |
1 |
|
|
Perfusion MRI |
|
|
|
|
CBF+CBV |
10 |
4 |
6 |
|
CBF-only |
3 |
2 |
1 |
|
CBV-only |
2 |
2 |
0 |
|
Negative |
9 |
3 |
6 |
Table 3.
Comparison of diagnostic tools for cerebral hyperperfusion syndrome
|
Total |
Positive |
Negative |
Sensitivity (%) |
|
FLAIR |
24 |
24 |
0 |
100.00 |
|
ADC |
24 |
24 |
0 |
100.00 |
|
SPECT |
23 |
11 |
12 |
47.83 |
|
Perfusion MRI |
24 |
13 |
9 |
59.09 |
References
1. Benzel EC, Hoppens KD : Factors associated with postoperative hypertension complicating carotid endarterectomy. Acta Neurochir (Wien) 112 : 8-12, 1991    4. ujimura M, Inoue T, Shimizu H, Saito A, Mugikura S, Tominaga T : Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc Dis 33 : 436-445, 2012    5. Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T : Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 71 : 442-447, 2009   6. Fujimura M, Shimizu H, Mugikura S, Tominaga T : Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol 71 : 223-227; discussion 227, 2009   7. eschwind N, Levitsky W : Human brain: left-right asymmetries in temporal speech region. Science 161 : 186-187, 1968   8. Hayashi K, Horie N, Suyama K, Nagata I : Incidence and clinical features of symptomatic cerebral hyperperfusion syndrome after vascular reconstruction. World Neurosurg 78 : 447-454, 2012   9. Heros RC, Scott RM, Kistler JP, Ackerman RH, Conner ES : Temporary neurological deterioration after extracranial-intracranial bypass. Neurosurgery 15 : 178-185, 1984    10. Janigro D, West GA, Nguyen TS, Winn HR : Regulation of blood-brain barrier endothelial cells by nitric oxide. Circ Res 75 : 528-538, 1994   12. Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK : Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis 25 : 580-586, 2008    14. Nomura S, Yamaguchi K, Ishikawa T, Kawashima A, Okada Y, Kawamata T : Factors of delayed hyperperfusion and the importance of repeated cerebral blood flow evaluation for hyperperfusion after direct bypass for Moyamoya disease. World Neurosurg 118 : e468-e472, 2018   15. Ogasawara K, Inoue T, Kobayashi M, Endo H, Fukuda T, Ogawa A : Pretreatment with the free radical scavenger edaravone prevents cerebral hyperperfusion after carotid endarterectomy. Neurosurgery 55 : 1060-1067, 2004    16. Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, et al : Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 107 : 1130-1136, 2007   17. Ohta T, Tanaka H, Kuroiwa T : Diffuse leptomeningeal enhancement, “ivy sign,” in magnetic resonance images of moyamoya disease in childhood: case report. Neurosurgery 37 : 1009-1012, 1995  18. Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T : Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol 69 : 281-286; discussion 286-287, 2008   19. Pang CH, Lee SU, Lee Y, Kim WB, Kwon MY, Sunwoo L, et al : Prediction of hemorrhagic cerebral hyperperfusion syndrome after direct bypass surgery in adult nonhemorrhagic moyamoya disease: combining quantitative parameters on RAPID perfusion CT with clinically related factors. J Neurosurg 138 : 683-692, 2022   20. Piepgras DG, Morgan MK, Sundt TM Jr, Yanagihara T, Mussman LM : Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 68 : 532-536, 1988   21. Sato K, Yamada M, Kuroda H, Yamamoto D, Asano Y, Inoue Y, et al : Time-of-flight MR angiography for detection of cerebral hyperperfusion syndrome after superficial temporal artery-middle cerebral artery anastomosis in moyamoya disease. AJNR Am J Neuroradiol 37 : 1244-1248, 2016    22. Toga AW, Thompson PM : Mapping brain asymmetry. Nat Rev Neurosci 4 : 37-48, 2003    23. Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N : Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke 43 : 2610-2616, 2012   24. Uno M, Nakajima N, Nishi K, Shinno K, Nagahiro S : Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease--case report. Neurol Med Chir (Tokyo) 38 : 420-424, 1998   25. van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, et al : Cerebral hyperperfusion syndrome. Lancet Neurol 4 : 877-888, 2005   26. Yu J, Zhang J, Li J, Zhang J, Chen J : Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease: systematic review and meta-analysis. World Neurosurg 135 : 357-366.e4, 2020   27. Zhao WG, Luo Q, Jia JB, Yu JL : Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease. Br J Neurosurg 27 : 321-325, 2013  
|
|




















