Ozgıray, Husemoglu, Cınar, Bolat, Akınturk, Bıceroglu, and Kızmazoglu: The Effect of Preoperative Three Dimensional Modeling and Simulation on Outcome of Intracranial Aneursym Surgery
Abstract
Objective
Three-dimensional (3D) printing in vascular surgery is trending and is useful for the visualisation of intracranial aneurysms for both surgeons and trainees. The 3D models give the surgeon time to practice before hand and plan the surgery accordingly. The aim of this study was to examine the effect of preoperative planning with 3D printing models of aneurysms in terms of surgical time and patient outcomes.
Methods
Forty patients were prospectively enrolled in this study and divided into two groups : groups I and II. In group I, only the angiograms were studied before surgery. Solid 3D modelling was performed only for group II before the operation and was studied accordingly. All surgeries were performed by the same senior vascular neurosurgeon. Demographic data, surgical data, both preoperative and postoperative modified Rankin scale (mRS) scores, and Glasgow outcome scores (GOS) were evaluated.
Results
The average time of surgery was shorter in group II, and the difference was statistically significant between the two groups (p<0.001). However, no major differences were found for the GOS, hospitalisation time, or mRS.
Conclusion
This study is the first prospective study of the utility of 3D aneurysm models. We show that 3D models are useful in surgery preparation. In the near future, these models will be used widely to educate trainees and pre-plan surgical options for senior surgeons.
Key Words: 3D printing · Cerebral aneurysm · Surgical model.
INTRODUCTION
Subarachnoid haemorrhage (SAH) due to intracranial aneurysm (IA) rupture results in high mortality and morbidity rates. The risk of SAH due to IA rupture is 2% per year. The mortality rate of SAH is approximately 60% in the first 6 months [ 1- 3, 22]. Recently, surgical treatment of aneurysms has decreased, especially in some centres, due to the widespread use of endovascular modalities [ 13, 14]. However, surgery remains the first-line treatment for complex aneurysms not suitable for endovascular treatment and for anterior circulation aneurysms [ 21, 24]. Nowadays, in surgical training, clinical experience is traditionally gained through cadaver courses and by observing different procedures. Nevertheless, the cost of cadavers is gradually increasing for surgical training, and in several countries, obtaining cadavers is difficult. Furthermore, many hospitals and training centres offer several opportunities for neurovascular surgery and many types of surgical operations. A way of learning intracranial anatomy and neurovascular diseases is to use medical simulators for procedural training. With the help of simulation, young surgeons and surgeon candidates may combine theoretical knowledge with physical processes and surgical techniques.
Three-dimensional (3D) printers are advanced technological devices that convert 3D models, designed with the aid of computers, into real physical objects. Currently, 3D printing technology provides simulation from models obtained from medical imaging data as customised models, which are effective in developing surgical strategies and plans. Aneurysm simulation using 3D printing is especially important for training young neurosurgeons [ 11, 12, 17, 18, 24]. These models are useful in visualising aneurysm locations, sizes, and dimensions, as well as the direction of the aneurysm. It is also helpful in analysing the relationship between the aneurysm and the main artery. According to the literature, surgical planning has been previously performed using 3D aneurysm models; however, no prospective studies have been conducted regarding this topic [ 11, 12, 17, 18, 24]. This prospective study was conducted to compare patients who underwent preoperative planning performed using 3D modelling and 3D printing with those who did not undergo preoperative planning using the aforementioned techniques, in terms of surgical time and outcomes.
MATERIALS AND METHODS
In this study, the medical records of 47 patients with aneurysms who were treated between 2018 and 2019 in our clinic were evaluated. The Ethics Committee of Ege University approved this study (approval No. 193T/25). The demographic data of the patients; Glasgow coma scale (GCS) scores at admission, surgery, hospitalisation, and follow-up; postoperative modified Rankin scale (mRS) scores; and Glasgow outcome score were examined. The patients were clinically evaluated using the Hunt-Hess grading system, while radiological evaluation was performed using the Fisher grading system. Three patients with negative angiogram and four patients with multiple aneurysms were excluded, and 40 patients were finally enrolled in this study and were divided into two groups : group I (n=20) consisted of patients who did not undergo preoperative surgical planning using a 3D printer, and group II (n=20) comprised patients who underwent preoperative surgical planning using a 3D printer ( Figs. 1- 6). The study was terminated when both groups reached a total number of 20 patients per group. All surgical clippings of the aneurysms were performed by the same experienced neurovascular surgeon. Pterional craniotomy was performed in 37 patients, and anterior interhemispheric craniotomy was performed in three patients.
Creation of 3D models
Digital Imaging and Communication in Medicine images obtained from computed tomography angiography (CTA) (Siemens Healthcare, Forchheim, Germany) images were reconstructed to create 3D models. Multidimensional datasets (axial, coronal, and sagittal) were transferred to a 3D Slicer (version 4.8; Isomics, Inc., Cambridge, MA, USA), a reconstruction software. Reconstruction of the intracranial artery was performed using a 3D Slicer after format conversion and extraction procedures. Subsequently, a masking procedure was performed. Segmentation was performed to isolate the cranium and aneurysmal main vessel geometries as binary masks. The data were converted to the stereolithography (STL) format for production using 3D printers. These procedures were performed by the same neurosurgeon and engineer in the study group.
For the model, a 3D engineering software with the SLT file format was used for image processing of the targeted region. A Meshmixer (Autodesk Inc., San Francisco, CA, USA) was downloaded using the STL editing software for surface cleaning, mesh optimisation, smooth structure processing, and surface deformation removal on the created model. The model was then sliced using the Cura software (version 4.8.0; Ultimaker BV, Utrecht, Netherlands) to create G codes. After processing by various operations, the solid model was finalised by removing surface mistakes. The procedure settings were standardised as follows: extruder temperature, 215°C; room temperature, 24°C; primary layer height, 0.1 mm; and filling rate, 20%. The 3D models of the cranium and aneurysm were printed in double colour using Ultimaker 3 Extended (Ultimaker BV) and a dual extruder desktop 3D printer using polylactic acid as the main material in a 1 : 1 ratio. The printing procedure lasted 4-36 hours. The surgical team chose the best approach and the clipping procedure after working on the 3D model. The most favourable clips were prepared for surgery. Since our clinic is a reference center, most of our patients usually apply to us on the first day of bleeding time. In our clinic, hyperacute surgery is not performed except for re-bleeding. This saves us the time needed for 3D printing.
In three patients who underwent urgent surgery, only aneurysm modelling was performed; for the remaining patients, elective surgery was planned, and the lesion was modelled from the skull base. The models of the patients who underwent urgent surgery could only be examined perioperatively, whereas longer examination of the models of patients who underwent elective surgery was possible. After the printing process, the models were transported to our hospital in approximately 30 minutes.
Statistical analysis
The study data were statistically analysed using the Statistical Package for the Social Sciences (SPSS version 22.0; IBM Corp., Armonk, NY, USA). Data and median values of the data were processed and transferred to the statistical analysis program. The Mann-Whitney U-test was used to compare the mean differences, whereas the chi-squared test was used to compare ordinal and dichotomous variables. Statistical significance was set at p<0.05.
RESULTS
The mean patient age was 54.8±12.9 years (range, 26-79). Twenty-one patients (52.5%) were women and 19 (47.5%) were men. The average hospitalisation time was 14.6±12.9 days (range, 4-75). The mean GCS score was 13.7±2.6 (range, 5-15) ( Table 1) at admission to the clinic. SAH was observed in 30 patients (75%). Among the 40 patients, 21 had a history of smoking. The average aneurysm volume was 149.9±155.4 mm3 (range, 5.2-658.8). Time interval between the SAH ictus and surgery is 2.9±1.1 days for 16 patients at group I, 3.2±0.8 days for 14 patients at group II ( p=0.539). Furthermore, among the 40 patients, 23 (76.7%) were ≤2 according to the Hunt-Hess grading system, and 24 were ≥3 according to the Fisher grading system. In this series, the most common IA localisation was the middle cerebral artery (MCA) (n=18, 45%), followed by the anterior communicating artery (n=12, 30%).
All patients underwent surgical clipping. The average time of surgery was 170.0±16.5 minutes (range, 150-210) in group I and 134.0±24.5 minutes (range, 90-180) in group II. The difference was statistically significant between the two groups (p<0.001). However, no significant differences in the initial GCS score (p=0.229) or hospitalisation time (p=0.838) were observed between the two groups.
In group II, in the three patients who had to be operated urgently, only aneurysm modelling was performed. Postoperatively, 11 patients (five in group I and six in group II) showed signs of vasospasm, and six patients had ischemia. Only one patient in each group had wound problems but were treated without the need for additional surgery.
Postoperatively, the mRS score was used to assess the status of the patients ( Tables 2 and 3). The mean follow-up duration of the patients was 12.1±1.9 months (range, 8-15). Only six patients had transient neurological deficits in the early postoperative period. Furthermore, 35 patients were discharged without any complications. However, 11 patients developed vasospasm; one of them was GCS 3 and grade V on the World Federation of Neurological Surgeons grading system (before surgery), one patient had cerebrospinal fluid leakage, and five patients had pneumonia. One patient died in each group. IA rupture is a disturbing condition for surgeons to consider even during preoperative planning. It can have dramatic effects on the morbidity and mortality of the patients. In this series, it occurred in three patients in group I, whereas only one patient in group II had IA rupture.
DISCUSSION
SAH caused by a ruptured IA remains a significant cause of morbidity and death, and despite the rapid development of endovascular treatment modalities, microsurgical clipping of IAs is a long-lasting, reliable treatment with a low recurrence rate [ 4, 5, 7, 10, 23]. Preoperative preparation with the evaluation of the angiograms, as well as the surgical experience of the surgeon, is important for successful IA clipping. In this study, the results of patients with personalised 3D aneurysm models were compared with those of the non-model group.
IA surgical training consists of cadaver model simulation and radiological 3D models; however, 3D solid model simulations have recently been used [ 9, 11, 12, 18, 24, 25]. 3D printing is superior to the other two methods in terms of obtaining information from patients. Personalised models are used in all surgical fields, not only in neurosurgery. In particular, in cranioplasty operations, the advantage of these models is indisputable [ 16]. A surgeon’s understanding of pathology by seeing and touching is superior to seeing only. 3D models have different timing and costs in solid model production owing to the type of 3D printer. Mashiko et al. [ 11], using colour ink-jet printers, have produced models in approximately 3 days. Ploch et al. [ 17] have printed silicon brain models in 24 hours, whereas Wang et al. [ 24] have printed cranium and aneurysm models in approximately 13-22 hours. In this study, using fused deposition modeling-type printers, the models were printed in 4-36 hours. Ryan et al. [ 18] simulated the clipping techniques for young neurosurgeons using 3D models. After the training of aneurysm clipping, 14 residents concluded that this simulation is beneficial in terms of education [ 18]. Wang et al. [ 24] compared six aneurysm models to bone and aneurysm models using computed tomography angiography; both were found to be useful. In a different study by Wang et al. [ 25], eight MCA aneurysms were modelled, and a survey was performed. Although the models were found to be useful, the thickness of the aneurysmal wall was not composed well enough [ 25]. Mashiko et al. [ 11] developed three unruptured MCA bifurcation aneurysm models using a 3D printer. The skull base, dura mater, arachnoid mater, cerebrum, arteries, and veins were modelled. Using this 3D model, a hollow silicon model was produced, and clip application was performed, which was shown to be useful for training young neurosurgeons [ 11]. In our study, with the help of surgical models, inexperienced surgeons in the surgical team understood the surgical planning more easily. With clip preparation beforehand, the surgery time was significantly reduced in both groups. This proves that in the future, surgical simulation will be further developed to decrease morbidity and mortality. In this study, the shorter surgery time in the 3D model group was found to be statistically significant. The anaesthesia time, medications, blood transfusions, and risk of infections will also be reduced. In the literature, a shorter surgery time is related to low infection and morbidity rates [ 10, 25]. Recently, clipping and coiling have been compared in all randomised studies. The International subarachnoid aneurysm trial has claimed that endovascular treatment is superior to clipping in the first year; however, in follow-ups, recurrence of haemorrhage was significantly higher in coiled patients [ 14]. Furthermore, the methodology of the trial has been heavily criticised after being published [ 6]. In the Barrow Ruptured Aneurysm Trial (BRAT) study, no difference in morbidity was observed between the treatment groups, and no re-haemorrhage was observed in patients who underwent surgical clipping [ 20, 21]. The recent BRAT study has revealed that surgical clipping remains valuable in treating aneurysms. In this study, vessel models were produced using solid models; hence, assessing the vessel wall thickness was difficult. Determining the relationship between the cranial nerves on CTA is difficult. Since perforated anterior communicating arteries are submillimetric, they could not be shown in the solid model, and this was the most important limitation of this methodology. This caused us to be unprepared for millimetre perforators coming out of the aneurysm during the real surgeries. Age is important for prognosis; in this study, the prognosis was worse in patients aged >50 years [ 26]. Nanda et al. [ 15] did not show the effects of age on prognosis in patients with unruptured aneurysms. The aneurysm occlusion rate was 94% in these patients after surgical clipping [ 15]. In a study by Jabbarli et al. [ 8], in patients with unruptured aneurysms, the morbidity rate was 2.5-8.5%, the mortality rate was 0-3.2%, and the postoperative complication rate was 25%. Song et al. [ 19] have shown that in patients with unruptured aneurysms, the morbidity rate was 0.5%, the mortality rate was 0.3%, and the postoperative complication rate was 2.8%. In this series, 10 patients (25%) had unruptured aneurysms.
CONCLUSION
Preoperative studies were conducted using one-to-one intracranial vessel modelling with the help of 3D printers, and a prospective comparison was made for the first time in the literature. Recently, 3D printers have been widely used in medical sciences; using this technology in neurosurgical practice with proper modifications and adaptation shows better results in terms of time and morbidity not only for aneurysms but also for tumour vascularization and spinal surgery. In order to exclude other factors, comparative study should be done only in the unruptured cerebral aneurysm group.
Fig. 1.
A : Computed tomography (CT) image shows a partially thrombosed aneurysm with a wide neck (arrow) at the left proximal A1 level in a 49-year-old female patient. B : Digital subtraction angiography (DSA) image shows saccular aneurysms at the level of the left proximal A1 and anterior communicating artery (arrows). C : After stent and coil embolization of the proximal A1 and anterior communicating artery aneurysms (arrows). D : On follow-up DSA, the anterior communicating artery aneurysm (white arrow) showed an increase in size. E : The distal end of the stent does not appear to cover the anterior communicating artery aneurysm (arrow). CT angiography after (F) clipping of the anterior communicating artery aneurysm. 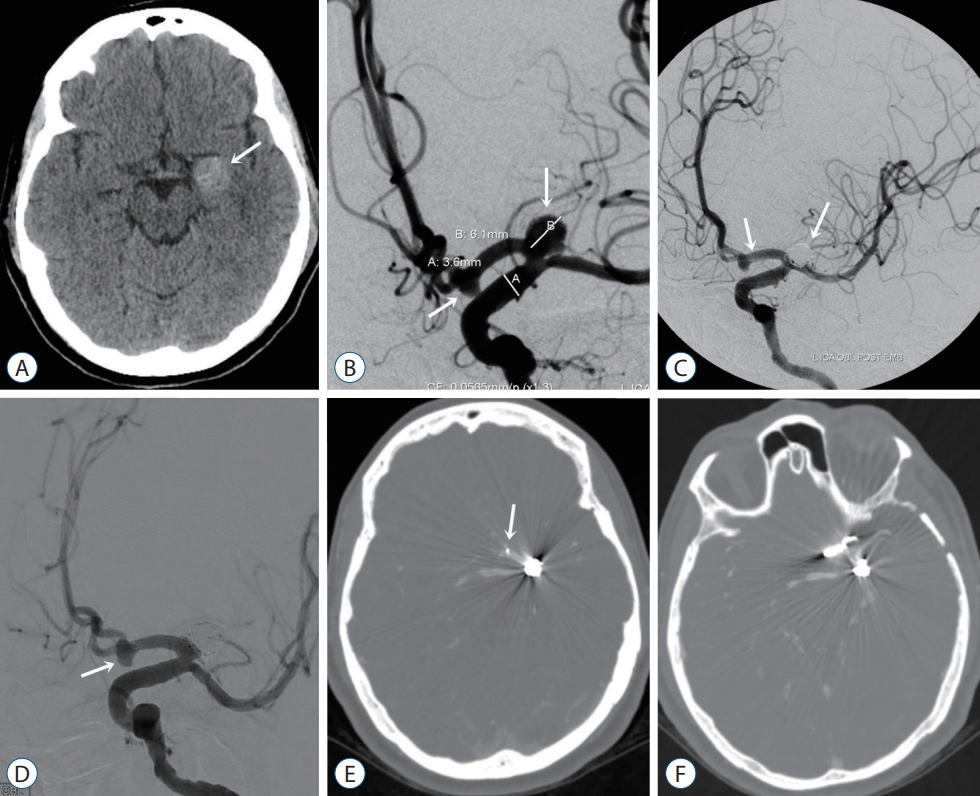
Fig. 2.
Patient-specific three-dimensional model. A : Isotropic view of a solid model in Meshmixer (Autodesk Inc., San francisco, CA, USA). B : Using Meshmixer to make a burr hole. C : Using Meshmixer for mini-craniotomy 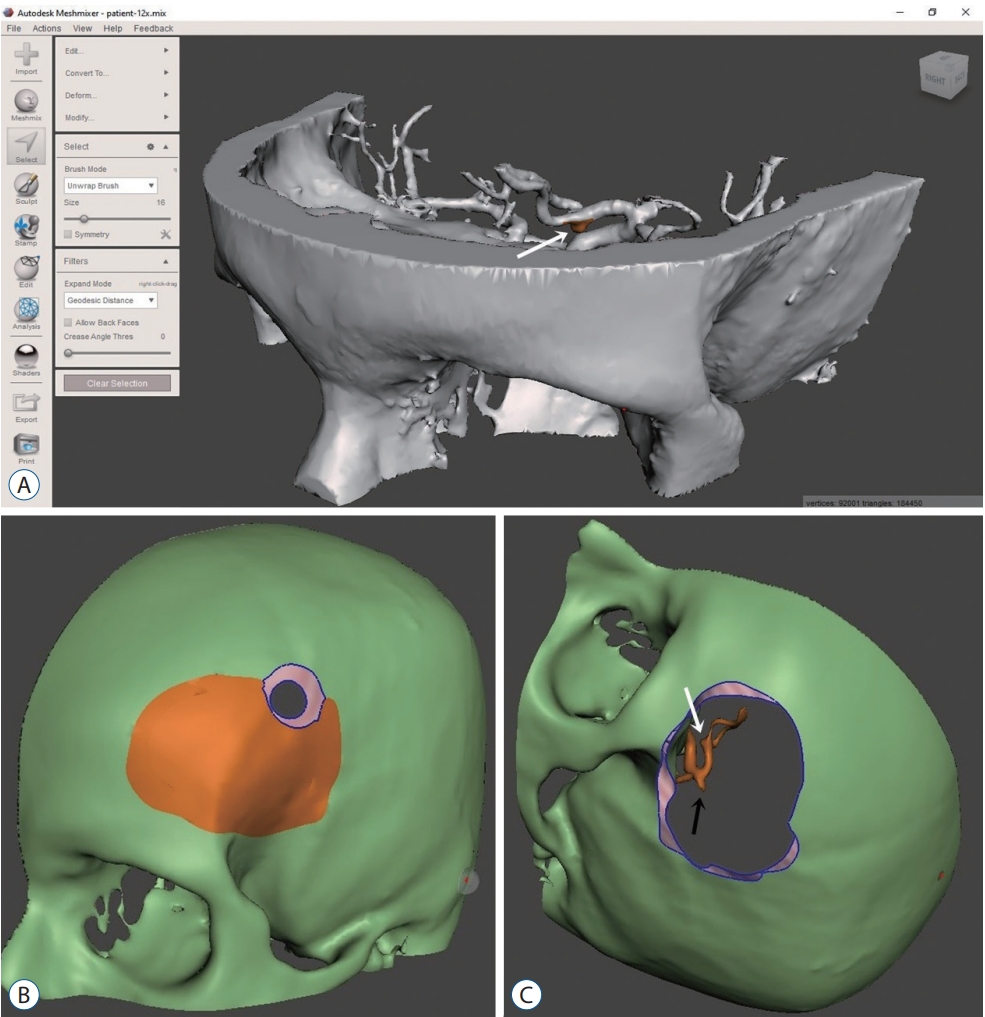
Fig. 3.
A : Three-dimensional (3D) printed Aneurysm model fixed with Mayfield head holder. B and C : Craniotomy was done and top view after printing of an anterior communicating artery aneurysm, (D) on the 3D printed model, clipping angle was simulated via left supraorbital approach. E : Intraoperative images still depicting a residual anterior communicating artery aneurysm before and (F) after clipping. ICA : internal carotid artery. 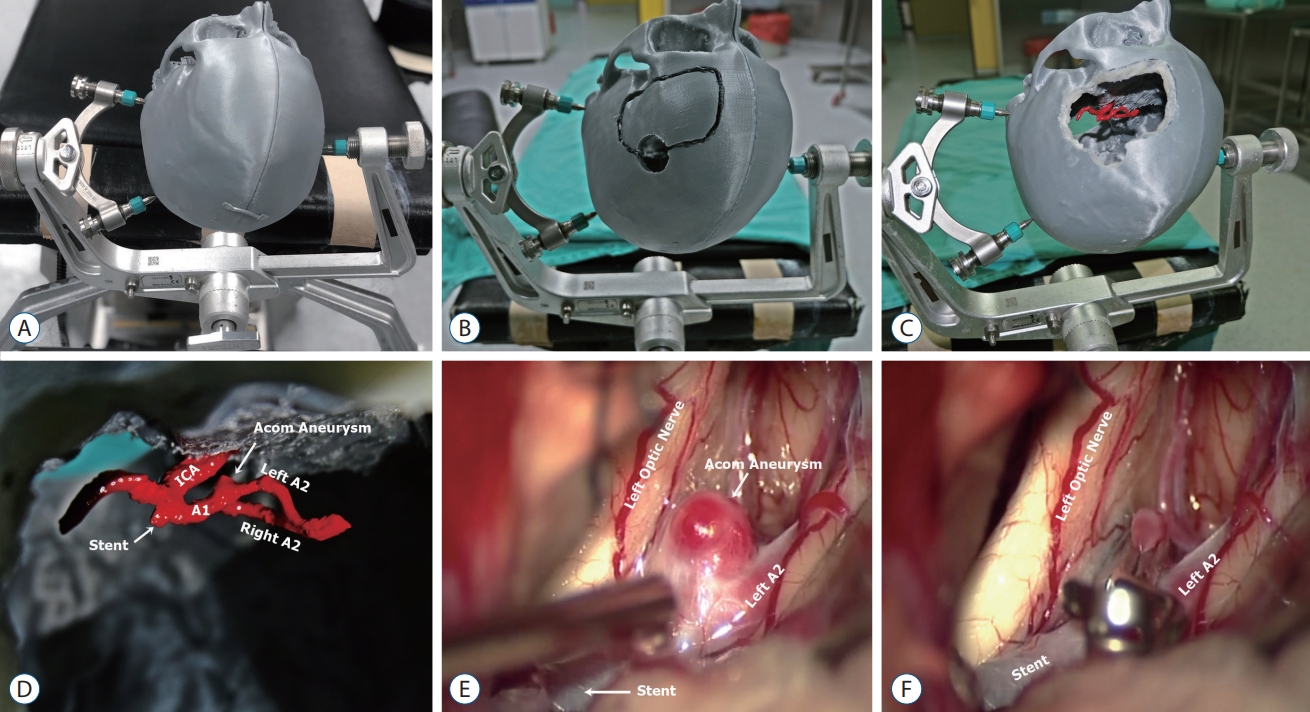
Fig. 4.
A 42-year-old male patient had an incidentally found right middle cerebral artery (MCA) bifurcation aneurysm. A : Digital subtraction angiography (DSA) depicting a right MCA aneurysm with a wide neck (arrow). B : Computed tomography (CT) image after endovascular treatment revealing the stent and coil (arrow). C : Two years after endovascular treatment, magnetic resonance angiography image shows newly developing microaneurysms at the level of the left MCA bifurcation (arrow). D : After endovascular treatment of left MCA aneurysms. The stent is depicted on the CT image (arrow). E : Four years after endovascular treatment, the control DSA image shows a newly developed saccular aneurysm at the level of the anterior choroidal artery (arrow). F : The saccular aneurysm was clipped via the left pterional approach. On postoperative CT, the aneurysm clip is depicted (arrow). 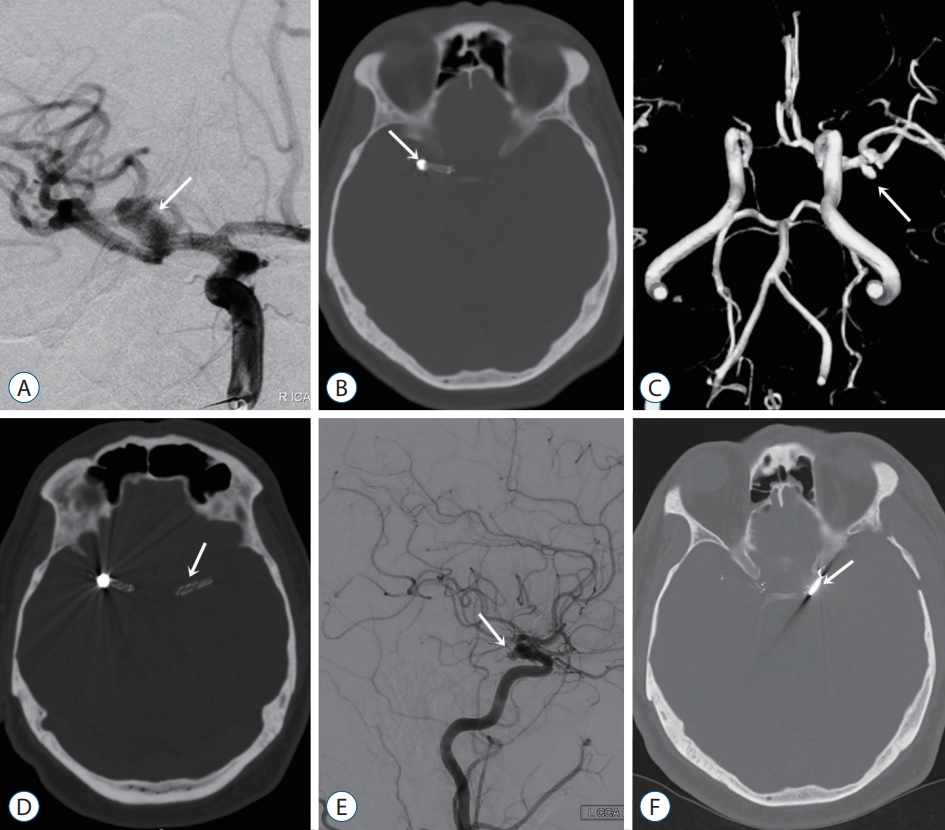
Fig. 5.
A and B : Computed tomography angiography images was used to reconstruct multiple aneurysms (arrows) model of the patient. C and D : Showing three-dimensional printed model of the aneurysms (arrows). 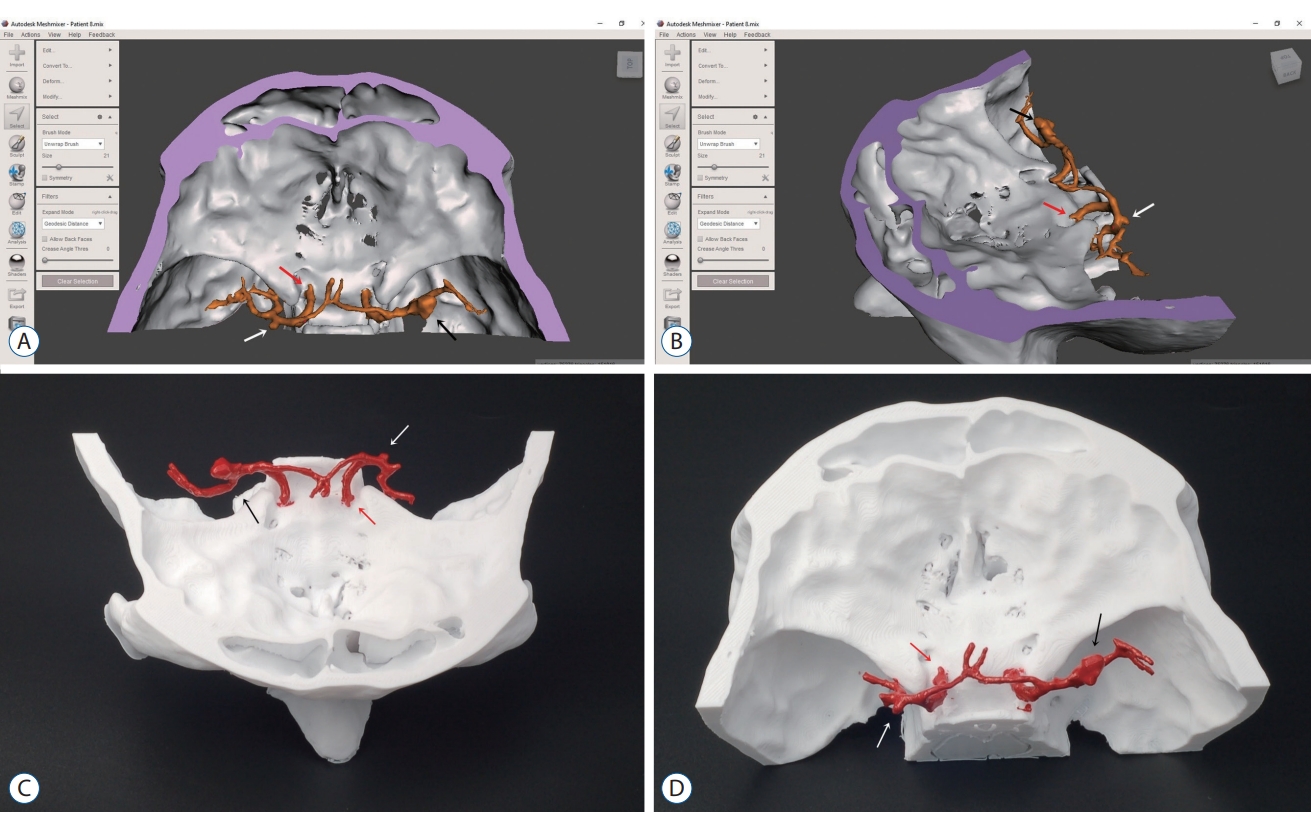
Fig. 6.
A left anterior choroidal artery aneurysm (white arrow) is observed before (A) and after clipping (B). Doppler ultrasonography is routinely employed during all aneurysm surgeries. In Fig. 3B, the patency of the parent artery is confirmed. 
Table 1.
Demographics and clinical characteristics of patients treated for aneurysms
|
Variable |
Total |
Group I |
Group II |
p-value |
|
Age (years) |
54.8±12.9 (26-79) |
55.9±13.9 (26-73) |
53.6±11.9 (29-79) |
0.350 |
|
Operation time (minutes) |
152.0±27.5 (90-210) |
170.0±16.5 (150-210) |
134.0±24.5 (90-180) |
<0.001 |
|
Patient hospital stay (days) |
14.6±12.9 (4-75) |
16.4±16.4 (4-75) |
12.9±8.0 (4-34) |
0.838 |
|
Follow-up (months) |
12.1±1.9 (8-15) |
11.7±2.1 (8-16) |
12.5±1.7 (9-16) |
0.248 |
|
GCS score |
13.7±2.6 (5-15) |
13.5±2.8 (5-15) |
14.0±2.4 (6-15) |
0.229 |
|
Aneurysm volume (mm3) |
149.9±155.4 (5.2-658.8) |
150.0±147.4 (5.2-456.7) |
149.8±165.1 (14.0-658.6) |
0.927 |
Table 2.
Outcomes in patients treated for aneurysms
|
Variable |
Total (n=40) |
Group I (n=20) |
Group II (n=20) |
p-value |
|
Sex |
|
|
|
0.342 |
|
Female |
21 (52.5) |
12 (60.0) |
9 (45.0) |
|
|
Male |
19 (47.5) |
8 (40.0) |
11 (55.0) |
|
|
Smoking |
21 (52.5) |
9 (45.0) |
12 (60.0) |
0.342 |
|
Diabetes mellitus |
3 (7.5) |
2 (10.0) |
1 (5.0) |
0.548 |
|
Hypertension |
24 (60.0) |
12 (60.0) |
12 (60.0) |
1.000 |
|
SAH |
30 (75.0) |
16 (80.0) |
14 (70.0) |
0.465 |
|
Aneurysm location |
|
|
|
0.125 |
|
Acom |
12 (30.0) |
6 (30.0) |
6 (30.0) |
|
|
Pcom |
3 (7.5) |
0 (0.0) |
3 (15.0) |
|
|
ICA |
4 (10.0) |
1 (5.0) |
3 (15.0) |
|
|
MCA |
18 (45.0) |
10 (50.0) |
8 (40.0) |
|
|
Pericallosal |
3 (7.5) |
3 (15.0) |
0 (0.0) |
|
|
mRS |
|
|
|
0.401 |
|
1 |
27 (67.5) |
14 (70.0) |
13 (65.0) |
|
|
2 |
6 (15.0) |
3 (15.0) |
3 (15.0) |
|
|
3 |
2 (5.0) |
0 (0.0) |
2 (10.0) |
|
|
5 |
1 (2.5) |
0 (0.0) |
1 (5.0) |
|
|
6 |
4 (10.0) |
3 (15.0) |
1 (5.0) |
|
|
GOS score |
|
|
|
0.494 |
|
1 |
5 (12.5) |
3 (15.0) |
2 (10.0) |
|
|
3 |
2 (5.0) |
0 (0.0) |
2 (10.0) |
|
|
4 |
5 (12.5) |
3 (15.0) |
2 (10.0) |
|
|
5 |
26 (70.0) |
14 (70.0) |
14 (70.0) |
|
|
Intraoperative rupture |
4 (10.0) |
3 (15.0) |
1 (5.0) |
|
|
Complications |
|
|
|
|
|
Infarcts |
6 (15.0) |
3 (60.0) |
3 (75.0) |
|
|
Pneumonia |
2 (5.0) |
1 (20.0) |
1 (25.0) |
|
|
CSF fistula |
1 (2.5) |
1 (20.0) |
0 (0.0) |
|
|
Vasospasm |
11 (27.5) |
5 (25.0) |
6 (30.0) |
|
|
Exitus |
2 (5.0) |
1 (5.0) |
1 (5.0) |
|
Table 3.
WfNS, fisher and Hunt-Hess gradings in patients with SAH
|
Variable |
Total |
Group I |
Group II |
p-value |
|
SAH |
30/40 |
16/20 |
14/20 |
|
|
WFNS grade |
|
|
|
0.784 |
|
1 |
16 (53.3) |
8 (50.0) |
8 (57.1) |
|
|
2 |
6 (20.0) |
4 (25.0) |
2 (14.3) |
|
|
3 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
4 |
5 (16.7) |
3 (18.8) |
2 (14.3) |
|
|
5 |
3 (10.0) |
1 (6.2) |
2 (14.3) |
|
|
Fisher grade |
|
|
|
0.493 |
|
1 |
2 (6.7) |
1 (6.3) |
1 (7.2) |
|
|
2 |
4 (13.3) |
1 (6.3) |
3 (21.4) |
|
|
3 |
3 (10.0) |
1 (6.3) |
2 (14.3) |
|
|
4 |
21 (70.0) |
13 (81.1) |
8 (57.1) |
|
|
Hunt-Hess grade |
|
|
|
0.752 |
|
1 |
3 (10.0) |
2 (12.5) |
1 (7.2) |
|
|
2 |
20 (66.7) |
10 (62.5) |
10 (71.4) |
|
|
3 |
1 (3.3) |
1 (6.2) |
0 (0.0) |
|
|
4 |
6 (20.0) |
3 (18.8) |
3 (21.4) |
|
References
1. Bakker NA, Metzemaekers JD, Groen RJ, Mooij JJ, Van Dijk JM : International Subarachnoid Aneurysm Trial 2009: endovascular coiling of ruptured intracranial aneurysms has no significant advantage over neurosurgical clipping. Neurosurgery 66 : 961-962, 2010  2. Cavallo C, Safavi-Abbasi S, Kalani MYS, Gandhi S, Sun H, Oppenlander ME, et al : Pulmonary complications after spontaneous aneurysmal subarachnoid hemorrhage: experience from barrow neurological institute. World Neurosurg 119 : e366-e373, 2018   3. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al : Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43 : 1711-1737, 2012   4. Fotakopoulos G, Tsianaka E, Fountas K, Makris D, Spyrou M, Hernesniemi J : Clipping versus coiling in anterior circulation ruptured intracranial aneurysms: a meta-analysis. World Neurosurg 104 : 482-488, 2017   5. Hernesniemi J, Ishii K, Niemelä M, Kivipelto L, Fujiki M, Shen H : Subtemporal approach to basilar bifurcation aneurysms: advanced technique and clinical experience. Acta Neurochir Suppl 94 : 31-38, 2005   6. Hernesniemi J, Koivisto T : Comments on “The impact of the International Subarachnoid Aneurysm Treatment Trial (ISAT) on neurosurgical practice”. Acta Neurochir (Wien) 146 : 203-208, 2004    7. Hernesniemi J, Niemelä M : Clipping of a ruptured aneurysm with clot removal in one session: still gold standard of treatment. World Neurosurg 74 : 579-580, 2010   8. Jabbarli R, Wrede KH, Pierscianek D, Dammann P, El Hindy N, Özkan N, et al : Outcome after clipping of unruptured intracranial aneurysms depends on caseload. World Neurosurg 89 : 666-671.e1, 2016   9. Kimura T, Morita A, Nishimura K, Aiyama H, Itoh H, Fukaya S, et al : Simulation of and training for cerebral aneurysm clipping with 3-dimensional models. Neurosurgery 65 : 719-726; discussion 725-726, 2009   10. Lehecka M, Dashti R, Lehto H, Kivisaari R, Niemelä M, Hernesniemi J : Distal anterior cerebral artery aneurysms. Acta Neurochir Suppl 107 : 15-26, 2010   11. Mashiko T, Kaneko N, Konno T, Otani K, Nagayama R, Watanabe E : Training in cerebral aneurysm clipping using self-made 3-dimensional models. J Surg Educ 74 : 681-689, 2017   12. Mashiko T, Otani K, Kawano R, Konno T, Kaneko N, Ito Y, et al : Development of three-dimensional hollow elastic model for cerebral aneurysm clipping simulation enabling rapid and low cost prototyping. World Neurosurg 83 : 351-361, 2015   13. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS : The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the international subarachnoid aneurysm trial (ISAT). Lancet 385 : 691-697, 2015    14. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al : International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366 : 809-817, 2005   15. Nanda A, Patra DP, Bir SC, Maiti TK, Kalakoti P, Bollam P : Microsurgical clipping of unruptured ıntracranial aneurysms: a single surgeon’s experience over 16 years. World Neurosurg 100 : 85-99, 2017  16. Park SE, Park EK, Shim KW, Kim DS : Modified cranioplasty technique using 3-dimensional printed ımplants in preventing temporalis muscle hollowing. World Neurosurg 126 : e1160-e1168, 2019  17. Ploch CC, Mansi CSSA, Jayamohan J, Kuhl E : Using 3D printing to create personalized brain models for neurosurgical training and preoperative planning. World Neurosurg 90 : 668-674, 2016   18. Ryan JR, Almefty KK, Nakaji P, Frakes DH : Cerebral aneurysm clipping surgery simulation using patient-specific 3D printing and silicone casting. World Neurosurg 88 : 175-181, 2016   19. Song J, Kim BS, Shin YS : Treatment outcomes of unruptured intracranial aneurysm; experience of 1,231 consecutive aneurysms. Acta Neurochir (Wien) 157 : 1303-1311, 2015    20. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P, et al : Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J Neurosurg 132 : 771-776, 2019   21. Spetzler RF, Zabramski JM, McDougall CG, Albuquerque FC, Hills NK, Wallace RC, et al : Analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J Neurosurg 128 : 120-125, 2018   22. Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, et al : European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 35 : 93-112, 2013    23. Tulamo R, Frösen J, Hernesniemi J, Niemelä M : Inflammatory changes in the aneurysm wall: a review. J Neurointerv Surg 10( Suppl 1):i58-i67, 2018   24. Wang L, Ye X, Hao Q, Chen Y, Chen X, Wang H, et al : Comparison of two three-dimensional printed models of complex intracranial aneurysms for surgical simulation. World Neurosurg 103 : 671-679, 2017   25. Wang L, Ye X, Hao Q, Ma L, Chen X, Wang H, et al : Three-dimensional intracranial middle cerebral artery aneurysm models for aneurysm surgery and training. J Clin Neurosci 50 : 77-82, 2018   26. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al : Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362 : 103-110, 2003  
|
|






















