Bot, Shilong, Philip, Dung, Shitta, Kyesmen, Alfin, Houlihan, Preul, Ozoilo, and Binitie: Predictors of Outcome in Management of Paediatric Head Trauma in a Tertiary Healthcare Institution in North-Central Nigeria
Abstract
Objective
Trauma is a leading causes of death and disability in all ages. The aim of this study was to describe the demography and characteristics of paediatric head trauma in our institution and examine the predictors of outcome and incidence of injury related mortality.
Methods
We examined our institutional Trauma Registry over a 2 year period.
Results
A total of 1100 trauma patients were seen over the study period. Of the 579 patients who had head injury 99 were in the paediatric age group. Of the paediatric head trauma patients 79 had documented Glasgow coma score (GCS), 38 (48.1%), 17 (21.5%) and 24 (30.4%) had mild, moderate and severe head injury respectively. The percentage mortality of head injury in the paediatric age group was 6.06% (6/99). There is an association between mortality and GCS (p=0.008), necessity for intensive care unit (ICU) admission (p=0.0001), associated burns (p=0.0001) and complications such as aspiration pneumonia (p=0.0001). The significant predictors of outcome are aspiration (p=0.004), the need for ICU admission (p=0.0001) and associated burns (p=0.005) using logistic binary regression. During the study period 46 children underwent surgical intervention with extradural haematoma 16 (34.8%), depressed skull fracture 14 (30.4%) and chronic subdural haematoma five (10.9%) being the commonest indication for surgeries.
Conclusion
Paediatric head injury accounted for 9.0% (99/1100) of all trauma admissions. Majority of patients had mild or moderate injuries. Burns, aspiration pneumonitis and the need for ICU admission were important predictors of outcome in children with traumatic brain injury.
Key Words: Head injury ¬Ј Traumatic brain injury ¬Ј Outcome ¬Ј Paediatric ¬Ј Predictors ¬Ј Nigeria.
INTRODUCTION
Trauma is one of the leading causes of death and disability. Trauma affects the young population the most. Paediatric head trauma is by far less common compared to that seen in the young adults. However, a significant number of children sustain head injuries either from road traffic accidents or falls.
The outcome of head trauma to a large extent depends on the severity of the injury. Head injury is the most common cause of death in children following trauma in developed and developing countries [ 10]. In the work by Osifo et al. [ 11] in Benin, paediatric head injury accounted for 56% of all paediatric and adolescent trauma deaths. Globally the incidence of paediatric traumatic injury varies from one country to the other, most ranging between 47 and 280 per 100000 [ 6]. Traumatic brain injury (TBI) in children is a great burden globally. In the United States of America between 1997 and 2007 the Centre for Disease Control reported that 73276 children died from TBI [ 3, 5]. It is difficult to assess the long-term morbidity effect of TBI [ 3]. From the KidsвАЩ inpatient database, which had data from over 3000 hospitals in the United States of America, 29000 had TBI while 4907 had what was classified severe TBI with a mortality of 24.2% [ 3, 13]. The cost of care in the acute period (acute hospital cost) was $2.56 billion [ 3, 12].
MATERIALS AND METHODS
Institutional Ethical Clearance of Jos University Teaching Hospital was obtained for this study. The data used for this study was obtained from our hospital Trauma Registry. The patients reviewed were entered into the Registry between 1st January 2012 and 31st December 2013.
Our Registry is a prospectively gathered continuous database. All trauma patients requiring admission are included. Each patient had a trauma datasheet that was filled on admission and completed on discharge. This information was then transferred into an Epi Info spreadsheet. Patients were followed up until discharge or death. The data was stored in a stand-alone password protected computer and was analyzed using IBM SPSS statistical software (IBM, Chicago, IL, USA). The data of the operations was obtained from the operation register. All head trauma patients who died in the emergency department and those not admitted were excluded from the review.
The SPSS statistical software (IBM) was used to analyzed the data, chi square and fisherвАЩs Exact test was performed for determinants of outcome with p-value set at p=0.05 for level of significance. The predictors of outcome were assessed using Chi square and binary logistic regression.
RESULTS
One thousand one hundred trauma patients were admitted. Of these patients, 579 (52.6%) had sustained a head injury. There were 176 paediatric trauma patients of which 99 children (56.3%) were diagnosed with TBI. The prevalence of paediatric head injury in the trauma population of the patients in our institution that required admission is 99/1100 (9.0%) ( Table 1). There were 69 males and 30 females with a male to female ratio of 2.3 : 1. The age distribution of the patients is stated in Table 2. The most common cause of head trauma in our series was road traffic accidents encompassing 55 patients (63.2%), falls from a height in 20 patients (23.0%), and domestic accidents occurring in four patients (4.6%) ( Table 1). The majority of paediatric patients were classified to have a mild head injury with a Glasgow coma score (GCS) of 13-15 (48.1%), while 30.4% suffered from a severe head injury (GCS of 3-8). Moderate head injury, defined as a GCS of 9-12 accounted for 17.8%. Only 79 patients had documented GCS in the registry. The percentage mortality was 6.1% (6/99) ( Table 1). Four out of 24 patients with severe head injury died. In two mortalities, the GCS was not documented. No mortalities were recorded in patients with mild or moderate head injury. Five patients required intensive care unit (ICU) admission, of which three died ( Table 3). Three patients aspirated, out of which two died [ 5]. There was an association noted between mortality and GCS ( p=0.008), necessity for ICU admission ( p=0.0001), associated burns ( p=0.0001) and complications such as aspiration pneumonia ( p=0.0001) ( Table 3). The significant predictors of outcome identified were aspiration ( p=0.004), the need for ICU admission ( p=0.0001) and associated burns ( p=0.005) using logistic binary regression ( Table 4). Further evaluation using logistic regression using two independent variables at a time as seen in Table 5 showed similar findings. Multinomial logistic regression of with the reference category survived, showed an odds ratio of 7.620 (GCS score), 411.857 (ICU admission), 0.000 (aspiration), and 15.840 (burns) as seen in Table 6. During the study period 46 patients received operative intervention. 71.4% were male and 28.3% were female. The mean age of those operated was 10 years (28 days to 17 years). The indications for surgery were extradural haematoma in 16 (34.8%), depressed skull fracture in 14 (30.4%), chronic subdural haematoma in five (10.9%), compound skull fracture in three (6.5%), acute subdural haematoma in two (4.3%), and penetrating injuries in a further two patients (4.3%).
DISCUSSION
This study shows that paediatric head injury accounted for 99 out of 1100 (9.00%) trauma admissions over the study period of 2 years. There were approximately 49 patients seen per year during the study period. In the study by Odebode and Abubakar [ 10] 267 patients were seen over a period of 10 years, an average of 26 patients per year. In Udoh and Adeyemo [ 14], 127 children with paediatric head injury were seen over a 5-year period. In Nnadi et al. [ 9] in Calabar, 75 children with head injury were reviewed over a 3 and a-half year period. Chinda et al. [ 4] had 45 paediatric head traumas over a period of 2 years and 2 months. Emejulu and Shokunbi [ 7] had 52 patients in Nnewi over a period of 1 year. Comparatively, our study showed a greater incidence of paediatric TBI. While most of the studiesвАЩ incidence of head trauma per year was less than in our institution, the study by Emejulu and Shokunbi [ 7] was noted to be higher.
Etiology
Road traffic accident (63.2%) was the most common cause of head injury followed by falls from a height (23.0%) and domestic injuries (4.6%). Odebode and Abubakar [ 10] in a 10-year retrospective study reviewed 267 patients with paediatric head trauma. In this series, the most prevalent aetiologies were road traffic accident (64.5%) and falls (30.7%). In the study by Udoh and Adeyemo [ 14] in 2014 their prospective study had a total of 127 patients over a 5-year period. Road traffic accidents accounted for 67.7% of cases and fall in 14.0% [ 14]. Our study shows similar results. The relatively high incidence may be due to the cosmopolitan nature of the city and the favourable temperate climate, which allows for out-door activities. Also, the growth of fruit trees like mangoes and oranges makes it one of the risk factors for falls from heights.
The severity of head injury and other predictors of outcome
The majority of paediatric patients had mild head injury, i.e., 36 patients (49.3%), followed by severe head injury in 24 patients (32.9%) and moderate head injury in 13 patients (17.8%). The sum of mild and moderate head injury accounted for 67.1% of children in our series. Most studies have shown that mild head injury is the commonest in children [ 2, 4, 7, 10]. It is noted that severe head injury has the highest rate of mortality and morbidity. The study by Udoh and Adeyemo [ 14] showed that severe head injury was the most prevalent group managed by their neurosurgical department, but it should be noted that mild head injury with normal neuro-imaging was excluded. In the study by Odebode and Abubakar [ 10] in Ilorin, age and coma score were the most accurate predictors of outcome. Similar to their study we found that a low GCS and invariably the need for intensive care admission had a significant association with mortality. In addition, further evaluation showed that necessity for ICU admission, aspiration pneumonia and associated burns were important predictors of mortality. In light of this knowledge, prevention of aspiration pneumonitis should be a key aim in the management of children with head injury. Hence all children with severe head injury should be intubated according to standard guidelines of management despite our limited resources and manpower in a developing nation. It also highlights the need for adequate training amongst the physicians and nursing staff in securing and managing the paediatric airway in an emergency situation Children with TBI and associated burns should be managed in an ICU setting with their airway secured at an early stage. Comprehensive wound care, aggressive fluid and electrolyte resuscitation and prophylactic antibiotics should all be instituted. In our study the percentage mortality was 6.1% (6/99). In the study by Udoh and Adeyemo [ 14] 11 patients died resulting in a mortality rate of 8.7%, Agrawal et al. [ 2] had a mortality of 3/43 (7.0%), while in a study by Odebode and Abubakar [ 10] in Ilorin, 38 patients died giving a percentage mortality of 14.2%. There are some other studies where the mortality rate was more than 10.0% [ 1, 4, 7, 15]. Our mortality rate for paediatric head trauma which is comparable to other good centers could be attributable to the organizationвАЩs infrastructure where the services are divided into general and paediatric trauma care. The multidisciplinary team encompasses a dedicated trauma team, a neurosurgical team and a paediatric surgery team that provides cover 24 hours a day. This ensures that the patients get optimum care.
ICU and high dependency unit (HDU) in a developing nation
Our institution is a tertiary health center in North-Central Nigeria. It is the neurosurgical center caring for the host state (Plateau State) and three other states, which include Bauchi, Nasarawa and Taraba State. This hospital has 630 beds. However, we have only six ICU beds with four ventilators caring for surgical, medical and paediatric patients. Not all patients requiring ICU care get admitted to it. As a way to cope with the high demand for ICU space the hospital has a HDU that is mainly run by the neurosurgical team. Patients with head injury having a GCS of 12 or below are usually admitted to the HDU. Those who have a GCS of 8 or below are intubated. The HDU has ICU trained nurses, even though ventilators and facilities for continuous monitoring are not available. Due to the constraints and high demand for the ICU care, as well as personal patient healthcare expense, only patients who require ventilator support or with haemodynamic instability are transferred to the ICU. Some patients with a GCS of 8 and below may have endotracheal intubation with oxygenation, without being connected to a ventilator as seen in Fig. 1. This allows maintenance of a patent airway and has shown favourable results in the prospectively collected data at our institution. This approach of endotracheal intubation without mechanical ventilation can be done in patients who have good respiratory effort with good oxygen saturation. We think that the development of the HDU with trained ICU nurses improves the patientвАЩs outcomes with TBI in developing countries where ICU facilities are limited (e.g., ventilators, multiparameter monitors, arterial blood gas [ABG] machines). This also will have a positive impact on the associated personal patient cost. We hope that in the near future our HDU will be able to have facilities for continuous monitoring and possibly ventilators. A simple algorithm has been developed by our unit to help in the management of head injury in low-income countries with limited intensive care facility ( Fig. 2).
Difference between our ICU, HDU and the regular wards
Our ICU has six beds with four ventilators. Each patient has facility for continuous monitoring of blood pressure, pulse rate, respiratory rate and oxygen saturation with ECG tracing. The vital signs are manually charted hourly. It has about one nurse to two patients ratio. About three physicians are attached to the unit in the daytime, but only one physician is available during the call hours, while simultaneously being responsible for patients requiring emergency surgical intervention. The limited number of the physician(s) (anaesthesiologist) during the call time is a major problem when there are patients that are in critical state that need close observation and intervention.
Our HDU is far from what is seen in most developed nations. It consists of a 9-bedded ward without continuous monitoring with vital signs taken hourly. Oxygen saturation is also assessed intermittently (spot check) using a pulse oximeter due to limited availability of pulse oximeters. The nurse to patient ratio is 1 to 3. Our regular wards have a nurse to patient ration of 1 : 10, where vital signs are taken 6 hourly.
All patients with a GCS of 3-8 are intubated (endotracheal) and either admitted to the ICU or HDU. Those who are breathing spontaneously and have good oxygen saturation are admitted to the HDU. These patients should have and endotracheal tube inserted with an oropharyngeal airway to prevent biting of the tube. An oropharyngeal airway (MayoвАЩs tube) only is not sufficient because the swallowing reflex is reduced in patients with severe head injury and the risk drowning in their saliva. In developed nations patients who have an impaired level of consciousness and a closed head injury with concerns of rising intracranial pressure (ICP), the standard of care would normally be to admit the patient to the ICU and insert an ICP monitoring probe. In our setting ICP probes and ABG machines are not available.
Challenges of using the 2019 consensus guideline for management of paediatric severe TBI in low resource nations
The third edition of the consensus guideline for management of severe TBI published in 2019, as part of the guideline for management of head injury by the Brain Trauma Foundation has a beautiful and detailed algorithm for the care of paediatric patients with severe TBI [ 8]. The algorithm states the first and second tier therapies. However, some of the recommendations may be difficult in resource poor settings. The components of the algorithm for first tier therapy include 1) baseline care, 2) ICP pathway, 3) herniation pathway, 4) cerebral perfusion pressure (CPP) pathway and 5) the brain tissue partial pressure of oxygen (PbrO 2) pathway [ 8]. Basic care includes endotracheal intubation, insertion of an ICP probe, mechanical ventilation, ABGs assessment aiming for a minimum PaCO 2 of 35 mmHg, normothermia <38oC, Haemoglobin >7 g/day, adequate intravascular volume managed via a central venous pressure, treatment of coagulopathy, 30o head up, and use of an anticonvulsant (phenytoin or leviteracetam) or continuous electroencephalography. Basic equipment for ventilation and rudimentary blood gas monitoring is in short supply in developing hospital settings. In resource poor regions, it is difficult to aspire and rely on guidelines and protocols, which do no mirror the facilities which are at our medical disposal. For example, in our, as well as many other developing nation institutions, we cannot adhere to the ICP pathway as we have a shortage of probes, external ventricular drains and hypertonic saline. We also cannot provide the optimal standard of care in the CPP pathway as vasopressors are not readily available and the equipment required for the PbrO 2 pathway is a rare commodity. Based on the unavailability of some of the equipment and expertise usually seen in the high-income nations, it is important to have a guiding algorithm like ours. Even though it appears basic, it will be of help and will improve outcomes in these patient groups. It is our recommendation, given the evidence portrayed, that Endotracheal intubation even in the absence of a ventilator should be recommended in resource poor settings. Patients with severe TBI with good respiratory effort are managed better with an endotracheal tube, a definitively secured airway, rather than attached to a mechanical ventilator with faulty batteries in regions where power outage is a common phenomenon and of the ability to routinely monitor ABG is sparse. The algorithm in Fig. 2 will undoubtedly help physicians in resource poor nations who struggle to provide the highest standard of care to high numbers of patients with severe TBI and limited ICU facilities.
Uniqueness of paediatric brain injury
A number of factors make paediatric TBI unique. These include the large head to body ratio compared to adults, more vulnerable brain cells due to the juvenile myelination and thin skull increasing the risk of parenchymal injury. There is also a higher rate of diffuse cerebral swelling and higher risk of raised ICP due to вАЬmalignant brain oedemaвАЭ.
Surgical management of paediatric head injury
From our study the three most common indications for operative intervention in the paediatric population are extradural haematoma 16 (34.8%), depressed skull fracture 14 (30.4%) and chronic subdural haematoma five (10.9%). Expertise in surgical management of these conditions should be competently achieved by training physicians at an early stage, particularly in resource poor regions where manpower may be deficient.
Limitations
The limitation of our study was deficits in the collection of full data sets on all TBI paediatric patients admitted to the service. This is however a large volume prospective study and gives comprehensive insight into the clinical, patient-related and socioeconomic burden of this condition on the regionвАЩs population.
CONCLUSION
This study showed that the prevalence of paediatric head injury amongst trauma patients was 9.0% in our institution. Prevention of complications especially aspiration pneumonia may significantly reduce mortality and improve the outcome of such patients. Aspiration, GCS, burns associated with head injury and the need for ICU admission are important determinants of outcome in children with paediatric head injury. The concept of a modified HDU can be used to reduce mortality in those with severe and moderate head trauma requiring ICU admission especially in a resource-constrained environment.
Acknowledgements
Thanks to the families with whom we worked.
We wish to also thank Mr. Kopse Seyilnen for helping with the statistical analysis.
Fig. 1.
Endotrachael intubation with oxygenation, without being connected to a mechanical ventilator. Used with permission from Barrow Neurological Institute, Phoenix, Arizona. 
Fig. 2.
Algorithm for the management of paediatric head injury. GCS : Glasgow coma score, HDU : high dependency unit, ICU : intensive care unit, ETT : endotracheal tube. 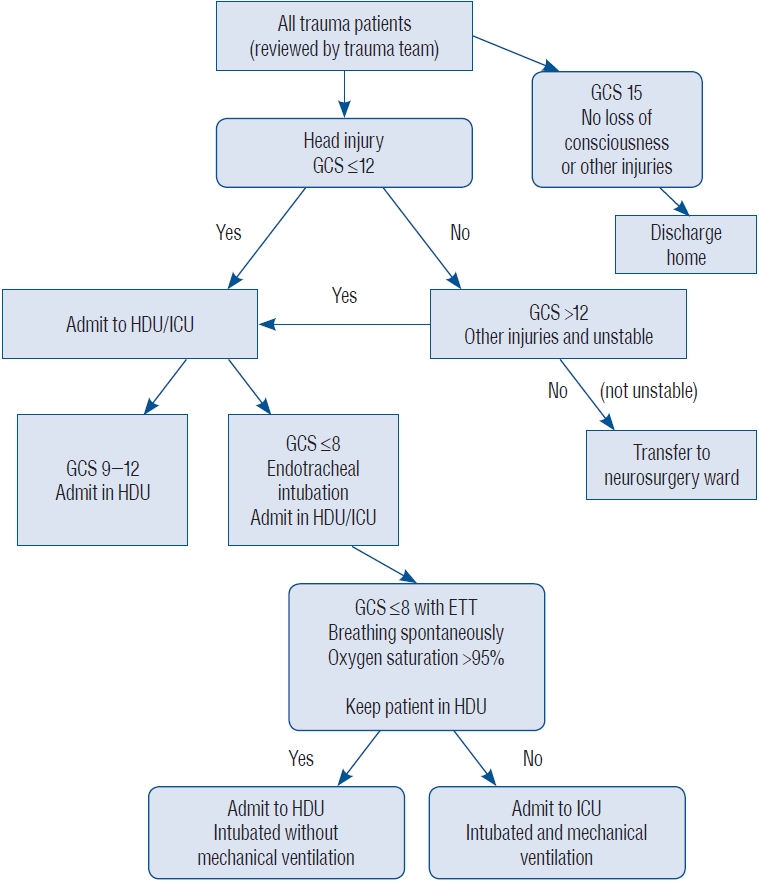
Table 1.
Summary of demography, aetiology, severity and outcome of paediatric TBI
|
Value |
|
Total trauma patients |
1100 |
|
Paediatric trauma |
176 |
|
Paediatric traumatic brain injury |
99 |
|
Age for paediatric TBI (years) |
8.87±4.96 |
|
Gender |
|
|
вАГMale |
69 (69.7) |
|
вАГFemale |
30 (30.3) |
|
Aetiology |
|
|
вАГRoad traffic accident |
55 (63.2) |
|
вАГFall from height |
20 (23.0) |
|
вАГDomestic accidents |
4 (4.6) |
|
вАГAssociated burns |
4 (4.6) |
|
вАГStab/cut injury |
1 (1.2) |
|
вАГOthers |
3 (3.4) |
|
Severity of TBI |
|
|
вАГMild |
38 (48.1) |
|
вАГModerate |
17 (21.5) |
|
вАГSevere |
24 (30.4) |
|
вАГTotal |
79 (100.0) |
|
Surgery |
|
|
вАГExtradural haematoma |
16 (34.8) |
|
вАГDepressed skull fracture |
14 (30.4) |
|
вАГCSDH |
5 (10.9) |
|
вАГCompound skull fracture |
3 (6.5) |
|
вАГASDH |
2 (4.3) |
|
вАГPenetrating injury |
2 (4.3) |
|
вАГOthers |
4 (8.7) |
|
Survival |
|
|
вАГDied |
6 (6.1) |
|
вАГSurvived |
93 (93.9) |
Table 2.
Age distribution of paediatric patients with head injury
|
Age |
Value |
|
<1 month |
0 (0.0) |
|
1 to 12 months |
8 (8.1) |
|
1 to 5 years |
20 (20.2) |
|
6 to 10 years |
33 (33.3) |
|
11 to 15 years |
25 (25.3) |
|
16 to <18 years |
13 (13.1) |
|
Total |
99 (100.0) |
Table 3.
Assessment of key determinants in patient outcome
|
Variable |
Outcome
|
Total |
Chi-squared |
p-value |
|
Died |
Survived |
|
Aspiration pneumonia |
|
|
|
|
|
|
вАГYes |
2 (66.7) |
1 (33.3) |
3 (100.0) |
19.960 |
0.0001*
|
|
вАГNo |
4 (4.2) |
92 (95.8) |
96 (100.0) |
|
|
|
ICU admission |
|
|
|
|
|
|
вАГYes |
3 (60.0) |
2 (40.0) |
5 (100.0) |
12.484 |
0.0001*
|
|
вАГNo |
1 (2.7) |
36 (97.3) |
37 (100.0) |
|
|
|
вАГUnknown |
2 (3.5) |
55 (96.5) |
57 (100.0) |
|
|
|
Burn injury |
|
|
|
|
|
|
вАГYes |
2 (50.0) |
2 (50.0) |
4 (100.0) |
14.136 |
0.0001*
|
|
вАГNo |
4 (4.2) |
91 (95.8) |
95 (100.0) |
|
|
|
вАГTotal |
6 (6.1) |
93 (93.9) |
99 (100.0) |
|
|
|
GCS score |
|
|
|
|
|
|
вАГSevere |
4 (16.7) |
20 (83.3) |
24 (100.0) |
9.656 |
0.008*
|
|
вАГModerate |
0 (0.0) |
17 (100.0) |
17 (100.0) |
|
|
|
вАГMild |
0 (0.0) |
38 (100.0) |
38 (100.0) |
|
|
|
вАГTotal |
4 (5.1) |
75 (94.9) |
79 (100.0) |
|
|
Table 4.
Logistic regression showing predictor variables and outcome of treatment
|
Variable |
B |
SE |
Wald |
df |
Sig. |
Exp(B) |
95% CI for Exp(B)
|
|
Lower |
Upper |
|
Aspiration pneumonia |
3.829 |
1.327 |
8.325 |
1 |
0.004*
|
46.000 |
3.414 |
619.845 |
|
Constant |
-4.522 |
2.502 |
3.266 |
1 |
0.071 |
0.011 |
|
|
|
ICU admission |
3.818 |
1.085 |
12.376 |
1 |
<0.001*
|
45.500 |
5.424 |
381.710 |
|
Constant |
-4.223 |
1.918 |
4.850 |
1 |
0.028 |
0.015 |
|
|
|
GCS score |
17.600 |
3597.907 |
0.000 |
1 |
0.996 |
44.012E06 |
0.000 |
- |
|
Constant |
-15.991 |
3597.907 |
0.000 |
1 |
0.996 |
0.000 |
|
|
|
Burns |
-3.125 |
1.123 |
7.742 |
1 |
0.005*
|
0.044 |
0.005 |
0.397 |
|
Constant |
3.125 |
0.511 |
37.407 |
1 |
<0.001 |
22.750 |
|
|
Table 5.
Multistage logistic regression showing predictor variables and outcome of treatment
|
Variable |
B |
SE |
Wald |
df |
Sig. |
Exp(B) |
95% CI for Exp(B)
|
|
Lower |
Upper |
|
Aspiration pneumonia |
2.339 |
1.728 |
1.832 |
1 |
0.176 |
10.366 |
0.351 |
306.272 |
|
ICU admission |
3.138 |
1.222 |
6.600 |
1 |
0.010*
|
23.063 |
2.105 |
252.735 |
|
Constant |
-7.466 |
3.518 |
4.505 |
1 |
0.034 |
0.001 |
|
|
|
Aspiration pneumonia |
2.944 |
1.433 |
4.224 |
1 |
0.040*
|
19.000 |
1.146 |
314.971 |
|
GCS score |
16.962 |
3604.192 |
0.000 |
1 |
0.996 |
23.246E06 |
0.000 |
- |
|
Constant |
-20.599 |
3604.192 |
0.000 |
1 |
0.995 |
0.000 |
|
|
|
Aspiration pneumonia |
4.500 |
1.418 |
10.068 |
1 |
0.002*
|
90.000 |
5.586 |
1449.981 |
|
Burns |
-3.807 |
1.229 |
9.589 |
1 |
0.002*
|
0.022 |
0.002 |
0.247 |
|
Constant |
-5.193 |
2.552 |
4.142 |
1 |
0.042 |
0.006 |
|
|
|
ICU admission |
19.045 |
3055.384 |
0.000 |
1 |
0.995 |
186.707E06 |
0.000 |
- |
|
GCS score |
32.752 |
4278.372 |
0.000 |
1 |
0.994 |
1.675E+14 |
0.000 |
- |
|
Constant |
-68.539 |
9642.999 |
0.000 |
1 |
0.994 |
0.000 |
|
|
|
ICU admission |
3.275 |
1.185 |
7.638 |
1 |
0.006*
|
26.443 |
2.592 |
269.781 |
|
Burns |
-1.871 |
1.498 |
1.561 |
1 |
0.212 |
0.154 |
0.008 |
2.898 |
|
Constant |
-3.041 |
2.177 |
1.951 |
1 |
0.162 |
0.048 |
|
|
|
GCS score |
17.646 |
3682.460 |
0.000 |
1 |
0.996 |
46.106E06 |
0.000 |
- |
|
Burns |
-15.699 |
29359.487 |
0.000 |
1 |
1.000 |
0.000 |
0.000 |
- |
|
Constant |
-16.037 |
3682.460 |
0.000 |
1 |
0.997 |
0.000 |
|
|
Table 6.
Multinomial logistic regression showing predictor variables and outcome of treatment
|
Variable |
B |
SE |
Wald |
df |
Sig. |
Exp(B) |
95% CI for Exp(B)
|
|
Lower |
Upper |
|
Intercept |
-6.811 |
4.101 |
2.758 |
1 |
0.097 |
|
|
|
|
GCS score |
2.031 |
1.452 |
1.957 |
1 |
0.162 |
7.620 |
0.443 |
131.093 |
|
ICU admission |
6.021 |
3.509 |
2.945 |
1 |
0.086 |
411.857 |
0.425 |
399235.688 |
|
Aspiration |
-7.764 |
5.934 |
1.712 |
1 |
0.191 |
0.000 |
3.774E-009 |
47.780 |
|
Burns |
2.763 |
4.297 |
0.413 |
1 |
0.520 |
15.840 |
0.003 |
72033.184 |
References
1. Adelson PD, Kochanek PM : Head injury in children. J Child Neurol 13 : 2-15, 1998    2. Agrawal A, Agrawal CS, Kumar A, Lewis O, Malla G, Khatiwada R, et al : Epidemiology and management of paediatric head injury in Eastern Nepal. Afr J Paediatr Surg 5 : 15-18, 2008   3. Bell MJ, Adelson PD, Wisniewski SR, Investigators of the ADAPT Study : Challenges and opportunities for pediatric severe TBI-review of the evidence and exploring a way forward. Childs Nerv Syst 33 : 1663-1667, 2017    4. Chinda JY, Abubakar AM, Umaru H, Tahir C, Adamu S, Wabada S : Epidemiology and management of head injury in paediatric age group in North-Eastern Nigeria. Afr J Paediatr Surg 10 : 358-361, 2013   5. Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, et al : Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ 60 : 1-32, 2011
6. Dewan MC, Mummareddy N, Wellons JC 3rd, Bonfield CM : Epidemiology of global pediatric traumatic brain injury: qualitative review. World Neurosurg 91 : 497-509.e1, 2016   7. Emejulu JK, Shokunbi MT : Aetiological patterns and management outcome of paediatric head trauma: one-year prospective study. Niger J Clin Pract 13 : 276-279, 2010  8. Kochanek PM, Tasker RC, Bell MJ, Adelson PD, Carney N, Vavilala MS, et al : Management of pediatric severe traumatic brain injury: 2019 consensus and guidelines-based algorithm for first and second tier therapies. Pediatr Crit Care Med 20 : 269-279, 2019   9. Nnadi MO, Bankole OB, Fente BG : Epidemiology and treatment outcome of head injury in children: a prospective study. J Pediatr Neurosci 9 : 237-241, 2014    10. Odebode TO, Abubakar AM : Childhood head injury: causes, outcome, and outcome predictors. A Nigerian perspective. Pediatr Surg Int 20 : 348-352, 2004  11. Osifo OD, Iribhogbe PE, Ugiagbe EE : Epidemiology and pattern of paediatric and adolescent trauma deaths in a level 1 trauma centre in Benin city, Nigeria. Injury 43 : 1861-1864, 2012   12. Shi J, Xiang H, Wheeler K, Smith GA, Stallones L, Groner J, et al : Costs, mortality likelihood and outcomes of hospitalized US children with traumatic brain injuries. Brain Inj 23 : 602-611, 2009   13. Stanley RM, Bonsu BK, Zhao W, Ehrlich PF, Rogers AJ, Xiang H : US estimates of hospitalized children with severe traumatic brain injury: implications for clinical trials. Pediatrics 129 : e24-e30, 2012    14. Udoh DO, Adeyemo AA : Traumatic brain injuries in children: a hospitalbased study in Nigeria. Afr J Paediatr Surg 10 : 154-159, 2013   15. Vaghani G, Singh PK, Gupta DK, Agrawal D, Sinha S, Satyarthee G, et al : Outcome of patients with traumatic head injury in infants: an institutional experience at level 1 trauma center. J Pediatr Neurosci 8 : 104-107, 2013   
|
|


















