Choo, Kim, Kim, Jeon, Jung, Ha, Oh, Shim, Kim, Jung, Park, Kim, Kim, Kim, and Lee: The Unique Relationship between Neuro-Critical Care and Critical Illness-Related Corticosteroid Insufficiency : Implications for Neurosurgeons in Neuro-Critical Care
Abstract
The brain houses vital hormonal regulatory structures such as the hypothalamus and pituitary gland, which may confer unique susceptibilities to critical illness-related corticosteroid insufficiency (CIRCI) in patients with neurological disorders. In addition, the frequent use of steroids for therapeutic purposes in various neurological conditions may lead to the development of steroid insufficiency. This abstract aims to highlight the significance of understanding these relationships in the context of patient care and management for physicians. Neurological disorders may predispose patients to CIRCI due to the role of the brain in hormonal regulation. Early recognition of CIRCI in the context of neurological diseases is essential to ensure prompt and appropriate intervention. Moreover, the frequent use of steroids for treating neurological conditions can contribute to the development of steroid insufficiency, further complicating the clinical picture. Physicians must be aware of these unique interactions and be prepared to evaluate and manage patients with CIRCI and steroid insufficiency in the context of neurological disorders. This includes timely diagnosis, appropriate steroid administration, and careful monitoring for potential adverse effects. A comprehensive understanding of the interplay between neurological disease, CIRCI, and steroid insufficiency is critical for optimizing patient care and outcomes in this complex patient population.
Key Words: Critical illness · Adrenal insufficiency · Intracranial pressure · Traumatic brain injury · Hypotension.
BACKGROUND
Patients with neurological critical illness may suffer from hormone deficiencies for various reasons. To diagnose and understand this, it is essential to recognize the physiological characteristics of the hypothalamic-pituitary-adrenal (HPA) axis and administer suitable steroids. Critical illness-related corticosteroid insufficiency (CIRCI) should be considered whenever a patient presents with an unexplained decreased level of consciousness, persistent hemodynamic instability, hypoglycemia, electrolyte imbalance, or fever despite the use of inotropes, and an adrenocorticotropic hormone (ACTH) stimulation test or empiric steroid administration is recommended. However, indiscriminate steroid use may increase the risk of infection or gastrointestinal bleeding, so it is imperative to be cautious. In neurologically critical patients, steroids are not recommended for controlling intracranial pressure and cerebral edema but should be reserved for cases such as adrenal insufficiency or electrolyte abnormalities. In addition, drug-induced adrenal insufficiency may occur in patients who have received steroids for a long period of time, so care should be taken when reducing the dosage.
LITERATURE REVIEW
HPA axis
The hypothalamus, pituitary gland, and adrenal glands form a complex that directly or indirectly influences one another to secrete hormones and suppress hormone secretion through a feedback mechanism. Corticotropin-releasing hormone (CRH) secreted by the hypothalamus triggers the pituitary gland to secrete ACTH, which then prompts the adrenal cortex to secrete cortisol. When cortisol levels rise, hormone homeostasis is maintained by suppressing CRH secretion from the pituitary gland through a negative feedback mechanism. The HPA axis is a crucial neuroendocrine system in the brain that responds to stress, activates under stress, and plays a central role in regulating numerous homeostatic systems in the body, including cardiovascular, immune, metabolic, reproductive, and central nervous systems [ 14] ( Fig. 1). The adrenal cortex, activated through the HPA axis, uses cholesterol as a precursor to produce glucocorticoids (cortisol), mineralocorticoids (aldosterone), and adrenal androgens (dehydroepiandrosterone, DHEA). Mineralocorticoids act on Na+/K+ pumps in renal tubule epithelial cells and regulate electrolyte and water balance (Na and H 2O uptake and K secretion). DHEA is a reproductive hormone that plays a role in fetal development and as a sex hormone. Glucocorticoids are mainly involved in carbohydrate, fat, and protein metabolism and exhibit anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictor effects. Glucocorticoids’ anti-inflammatory and immunosuppressive actions involve inducing (anti-inflammatory response) or suppressing (inflammatory response) gene transcription by directly or indirectly acting on cytoplasmic glucocorticoid receptors in structural cells such as inflammatory leukocytes and epithelial cells. In other words, glucocorticoids exert their effects by up-regulating the transcription of anti-inflammatory genes and down-regulating the transcription of inflammatory genes by down-regulating cytokine and chemokine proteins, cell adhesion molecules, and enzymes involved in the inflammatory response [ 3, 15] ( Fig. 2). Under normal conditions, glucocorticoids are typically produced in the zona fasciculata of the adrenal cortex. Their secretion is stimulated by stress signals and influenced by circadian rhythms, with peak levels occurring in the early morning and the lowest levels at midnight. In plasma, about 75-90% of cortisol is bound to corticosteroid-binding globulin (CBG), while 10-15% is present as albumin-bound or unbound free form. The normal glucocorticoid concentration ranges from 4.0 to 22 µg/dL (6 to 8 a.m.). When the concentration exceeds 25 µg/dL, the ratio of albumin-bound and free cortisol increases, while the amount of CBG-bound cortisol remains unchanged. Glucocorticoids are metabolized primarily in the liver and kidneys [ 1].
Characteristics of steroid agents
Since the introduction of Prednisone in 1954, various types of steroid preparations have become available. However, different steroid products exhibit diverse mineralocorticoid and glucocorticoid potencies and half-lives. It is crucial to accurately identify these potencies to use steroids with the appropriate dosage and administration. Prednisone and prednisolone are among the most widely used steroid agents, commonly employed for their anti-inflammatory and immunosuppressive properties due to their high glucocorticoid activity and low mineralocorticoid response. Although similar to prednisone, methylprednisolone has a lower mineralocorticoid response and may be preferred when water retention and aldosterone effects should be avoided. Dexamethasone has minimal mineralocorticoid activity but significantly stronger and longer-lasting glucocorticoid action than prednisone. Because of its high glucocorticoid efficacy, long-term dexamethasone use can cause severe HPA axis suppression and adrenal insufficiency. Therefore, it is generally recommended for short-term use during acute phases. Cortisone and hydrocortisone have relatively weak glucocorticoid activity compared to other steroids and exhibit both mineralocorticoid and glucocorticoid activity, making them suitable for patients with adrenal insufficiency.
Fludrocortisone has much higher mineralocorticoid potency than glucocorticoid potency, so it is used to replace aldosterone in salt-wasting forms of Addison’s disease and congenital adrenal hyperplasia. The effects of each steroid agent are displayed in Table 1 [ 15], and the potencies of steroid agents equivalent to 5 mg of prednisone are provided in Table 2 [ 14, 19].
CIRCI
Definition
In 2008, the term “Critical Illness-Related Corticosteroid Insufficiency” was introduced by the Association of International Multidisciplinary Projects organized by the Society of Critical Care Medicine (SCCM) to describe damage to the HPA axis in critically ill patients [ 16]. CIRCI is a response caused by insufficient downregulation of inflammatory transcription factors through glucocorticoid-glucocorticoid receptors and is defined as inadequate cellular corticosteroid activity for critically ill patients. CIRCI is thought to occur in various acute conditions, including sepsis and septic shock, severe pneumonia, acute respiratory distress syndrome (ARDS), heart attack, head injury, trauma, burns, and after major surgery. It affects approximately 10% of hospitalized critically ill patients and occurs in about 20% of cases. CIRCI is known to occur in around 60% of patients with septic shock [ 1]. When CIRCI occurs, the increase in inflammatory and coagulation factors over time is associated with increased patient morbidity, longer ICU stays, and higher mortality rates.
Mechanism of CIRCI
Under normal conditions and in the absence of stress, cortisol is primarily secreted during the day according to the circadian rhythm by ACTH, and ACTH is secreted from the pituitary gland under the control of CRH secreted from the hypothalamus and arginine vasopressin. When cortisol secretion increases, it acts on the pituitary gland and hypothalamus as a negative feedback mechanism to suppress secretion. Additionally, most of the secreted cortisol exists bound to CBG, and about 10% of the remaining cortisol is in an unbound free form, which is available for use in the body [ 8]. In stressful situations (infection, trauma, burns, surgery, etc.), cortisol levels can be about six times higher than under normal conditions, depending on the severity of the illness, and the normal diurnal variation disappears, maintaining consistently high levels. These changes are induced by various physiological mechanisms, including an increase in ACTH and CRH, and a decrease in the negative feedback function of cortisol. ACTH is additionally stimulated by norepinephrine produced in the locus coeruleus in stress condition. Furthermore, there are receptors for damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) in the nerve endings of afferent fibers of the autonomic nervous system in damaged tissue to sense threats and activate the noradrenergic/CRH system. DAMPs and PAMPs can also induce cortisol synthesis by directly stimulating adrenal cortical cells possessing Toll-like receptors ( Fig. 3). In addition to systemic action, cytokines produced by inflammatory cells can increase peripheral cortisol levels and enhance the affinity of cortisol receptors for cortisol. This change in cortisol activity is considered an important adaptive mechanism regulating the inflammatory response ( Fig. 4) [ 1]. The anti-inflammatory action of cortisol is properly maintained by interacting with the inflammatory action that occurs under stress, effectively controlling the inflammatory response. However, if cortisol activity is reduced, continuous and excessive inflammation occurs, leading to tissue damage, necrosis, and disease progression. This inappropriate decrease in cortisol activity can be diagnosed as CIRCI.
A decrease in cortisol activity is frequently observed in critically ill patients, and this can be explained by two mechanisms: adrenal cortisol hormone synthesis disorder and tissue resistance to cortisol. Adrenal cortisol synthesis disorder is caused by damage to neuroendocrine cells. Conditions such as sepsis and septic shock can lead to necrosis and hemorrhage of adrenal tissue, as well as damage to the pituitary gland and thalamus due to stroke. Furthermore, nitric oxide secretion increases under stress conditions like sepsis, inhibiting neuronal cell death and ACTH synthesis. Also, fat and cholesterol storage in the adrenal cortex are reduced, leading to decreased cortisol synthesis, which requires these reactants.
Tissue cortisol resistance occurs when there is a decrease in glucocorticoid receptor alpha (GR-α) activity despite normal plasma cortisol concentrations. This resistance can be influenced by the cell’s response to cortisol due to changes in the location of GR-α (such as cell nucleus or mitochondria) or other related transcription factors. This information is summarized in Table 3 [ 2].
Clinical symptoms of CIRCI
The clinical symptoms of CIRCI, which can manifest in various ways, as shown in Table 4. Specifically, in intensive care unit patients, CIRCI should be suspected when a persistent altered mental state has no other identifiable cause, when hemodynamic instability occurs despite vasopressor use, or when symptoms like hypoglycemia, fever, and electrolyte imbalance persist despite corrective measures. In such cases, steroid administration should be considered [ 2].
Diagnosis of CIRCI
High-dose (250-µg) ACTH stimulation test : corticosteroid (adrenal) insufficiency can be diagnosed when cortisol levels are less than 18 μg/dL at 30 and 60 minutes after administering 250 μg of cosyntropin [ 2]. According to the 2008 guidelines, CIRCI can be diagnosed when the change in serum cortisol after administering 250 μg of cosyntropin is less than 9 μg/dL or when the total cortisol is <10 μg/dL at random [ 16]. The guideline raised questions about the effectiveness of the previous two methods and they are no longer recommended. According to the American Endocrine Society’s guidelines, the high-dose (250 μg) ACTH stimulation test (30- or 60-minute cortisol level less than 18 μg/dL) diagnoses primary adrenal insufficiency more effectively than other diagnostic tests [ 2]. However, in actual clinical practice, the ACTH stimulation test is used as an auxiliary tool rather than an absolute diagnostic tool, and diagnosis often depends on the clinician’s judgment based on the patient’s severity, symptoms, and condition. As a secondary method, the low-dose (1 μg) ACTH stimulation test and the improvement of hemodynamic instability after administration of hydrocortisone (50-300 mg) can be used, but it is known to have less diagnostic value than the high-dose (250 μg) ACTH stimulation test. Other diagnostic tests, such as plasma total cortisol, random plasma or serum cortisol, and salivary free cortisol, have not yet been clearly demonstrated. Also, routinely measuring ACTH (corticotropin) levels to diagnose CIRCI is not recommended.
General management of corticosteroid insufficiency
The treatment of CIRCI does not recommend using steroids in a standard dosage and regime for all patients with suspected adrenal insufficiency among critically ill patients. Guidelines also recommend its use according to different diseases and conditions such as sepsis, septic shock, and ARDS. As such, CIRCI treatment has not been clearly established, and treatment is often determined by the experience and judgment of the clinician depending on the clinical situations [ 10]. Sepsis : The use of steroids is not recommended in adult septic patients without shock [ 10]. Septic shock : The use of steroids is recommended for patients with septic shock who do not respond to fluid therapy and who require moderate to high doses of vasopressors. When steroids are used, long-term and low-dose administration (e.g., hydrocortisone IV <400 mg/day IV for more than 3 days at full dose) is preferred over high-dose and short-term administration in adult septic shock patients. Common steroid use is a 200 mg/day dose of hydrocortisone IV, with 50 mg intravenous bolus injection or continuous infusion every 6 hours. It is usually recommended that these medications be initiated after at least 4 hours of administration at doses of norepinephrine or epinephrine ≥0.25 mcg/kg/min [ 10]. ARDS : Steroid use is recommended for patients with early moderate to severe ARDS (PaO 2/FiO 2 <200 and within 14 days of onset). As a result of analyzing nine trials, steroid administration was associated with a significant reduction in inflammatory cytokine, duration of mechanical ventilation, and reduction in mortality in patients with mild and severe ARDS. In addition, a multicenter, randomized study published in 2020 showed that dexamethasone treatment in ARDS patients significantly decreased the duration of mechanical ventilation and mortality, and the rate of side effects was not significantly different [ 23]. In the 2017 SCCM guidelines, for patients with persistent ARDS, it is suggested to administer methylprednisolone at 1 mg/kg/day until the 7th day of onset (PaO 2/FiO 2 <200) and at 2 mg/kg/day during the later period (6 days after onset). After that, it is advised to gradually taper the dosage over 13 days. Methylprednisolone is recommended due to its high lung tissue permeability.
CIRCI in neuro-critical care
Epidemiological investigations of CIRCI in neurocritical patients are still insufficient. Studies examining the incidence of adrenal insufficiency in traumatic brain injury (TBI) or subarachnoid hemorrhage report that it occurs in about 25% to 50% of patients with TBI, often within about 48 hours after the initial trauma [ 7, 9, 18]. In the case of subarachnoid hemorrhage, it can occur in about 37.5% to 69% of cases in the follow-up period [ 4, 24]. Cortisol levels initially rise after subarachnoid hemorrhage, but return to normal levels after the acute phase. No association has been found between the severity of subarachnoid hemorrhage and cortisol levels [ 24]. In the chronic phase (several months to several years), adrenal insufficiency occurs in one-third of patients, and mortality rates are significantly higher in patients with CIRCI (40%) compared to those without [ 25]. Hypothalamic-pituitary dysfunction due to TBI, subarachnoid hemorrhage, and ischemic stroke has recently gained attention. Symptoms such as fatigue, difficulty concentrating, and depression are nonspecific and often remain undiagnosed or delayed. However, neuroendocrine dysfunction after brain injury may occur at a much higher prevalence than previously known [ 4, 24]. Patients with pituitary insufficiency can have potentially serious and sometimes life-threatening consequences, affecting morbidity, functional and cognitive outcomes, and impairing quality of life [ 13]. Therefore, these patients should be screened for hypopituitarism, and if suspected, each pituitary hormone should be evaluated individually, as various patterns of hormone deficiency may accompany it. The pathophysiology of hypopituitarism due to brain injury has not yet been fully elucidated, and several factors have been suggested. Brain damage can cause damage to the anterior and posterior lobes and pituitary stalk in the form of hemorrhage, ischemia, necrosis, and fibrosis [ 20]. Additionally, pituitary vascular injury can also be caused by portal pituitary infarction or pituitary stalk severing. A recent study suggests a possible interaction between autoimmunity and hypopituitarism after TBI, as the blood-brain barrier is destroyed due to brain damage, causing an immune response as brain proteins leak through it [ 21, 22]. As pituitary dysfunction due to TBI and cerebral infarction has recently been discovered, testing for pituitary-hypothalamic hormones is highly recommended not only in the early stages of injury but also in the long term [ 13]. In one literature, within 1-7 days after the onset of injury, a 250-μg ACTH stimulation test, insulin tolerance test, and early morning serum cortisol test were used to confirm the presence of adrenal insufficiency, and in the long term, additional adrenal insufficiency tests at 3-6 months and 1 year were recommended. Furthermore, thyrotropin releasing hormone - thyroid stimulating hormone thyroid axis, GnRH-luteinizing hormone/ollicle stimulating hormone gonadal axis, somatotroph axis, and posterior pituitary tests are also recommended.
Use of steroid in controlling increased intracranial pressure (IICP) and brain edema
Recently, the use of steroids to control ICP and cerebral edema in the neurocritical patients with TBI and stroke is not recommended. The Brain Trauma Foundation has not recommended the use of steroids in TBI patients to improve prognosis or reduce ICP. High-dose methylprednisolone in patients with severe TBI has been associated with increased mortality, and its use is not recommended [ 6]. Additionally, the 2019 American Heart Association and American Stroke Association (AHA/ASA) acute ischemic stroke guidelines state that due to insufficient evidence for the efficacy of steroid use and the potential for increased risk of infectious complications, the use of steroids for the treatment of cerebral edema is contraindicated [ 17]. The 2022 AHA/ASA ICH guidelines also announced that the use of steroids for ICP control does not show efficacy and is not recommended [ 11]. Therefore, the use of steroids (including mineralocorticoids) to control ICP and cerebral edema in neurocritical patients with brain trauma and stroke is only recommended when there are signs of adrenal cortical dysfunction or electrolyte abnormalities, such as hyponatremia.
Exogenous glucocorticoid-induced adrenal insufficiency (GI-AI)
GI-AI is one of the most dangerous side effects of steroid therapy, involving symptoms and adrenal crisis caused by cortisol deficiency due to insufficient adrenal function and cortisol reduction. Therefore, it is always important to be aware of the possibility of GI-AI when tapering and discontinuing long-term steroid therapy.
The cause of GI-AI is the suppression of the HPA axis due to exogenous steroid administration. The negative feedback mechanism caused by increased glucocorticoids from steroid treatment leads to a decrease in CRH synthesis and secretion in the hypothalamus and a decrease in ACTH secretion by the pituitary gland. Initially, this negative feedback suppresses the synthesis of pro-opiomelanocortin (POMC) in the hypothalamus, reducing ACTH production. In the long term, atrophy of corticotroph cells and Crooke cells occurs due to decreased pituitary stimulation. The decrease in ACTH secretion can lead to a reduction in cortisol and androgen production in the adrenal cortex and atrophy of the zona fasciculata and reticularis ( Fig. 5). In a state the adrenal cortex is atrophied, suddenly stopping steroid treatment prevents cortisol secretion, leading to a persistent cortisol deficiency. Typically, after short-term glucocorticoid treatment, stopping the medication first results in recovery of ACTH secretion, followed by CRH, and eventually cortisol and androgen. However, in patients who have received long-term steroid treatment, a rapid and significant recovery in ACTH is observed, but cortisol secretion can be severely reduced for an extended period if adrenal atrophy has occurred. In patients with reduced cortisol secretion, an adrenal crisis can occur due to acute stress stimuli such as infection or surgery, putting the patient’s life at risk.
GI-AI patients may present with a range of cortisol deficiency symptoms, from asymptomatic to serious symptoms like adrenal crisis. Commonly known symptoms include fatigue, dizziness, gastrointestinal symptoms (nausea, vomiting, diarrhea, etc.), weight loss, low blood pressure, and headaches. These symptoms can be non-specific, gradually onset, and may be confused with other causes. A meta-analysis conducted in 2016 reported that adrenal insufficiency occurred in about 37% of patients receiving systemic steroids, with a higher incidence in those receiving high doses and long-term use [ 5, 12]. Risk factors include daily administration for more than 2-4 weeks, divided doses multiple times per day, and administering at night. Therefore, clinicians must be aware of the recovery of normal cortisol secretion and the symptoms and risk factors of GI-AI to safely discontinue steroid treatment.
PREVENTION OF GI-AI
To avoid adrenal insufficiency, it is recommended to use short-term, low-dose steroids whenever possible and to administer once daily for intermediate and long-acting steroids. Additionally, it is advised to avoid administration at bedtime [ 19]. In cases where steroid treatment lasts less than 2 weeks, adrenal insufficiency is generally unlikely, and tapering is not necessary. However, a tapering protocol is needed for those treated for more than two weeks. For initial high doses (>20 mg prednisolone), a reduction of 5-10 mg (daily 1-2 mg) per week is recommended, and for intermediate and low doses (<20 mg prednisolone), a reduction of 1-2.5 mg per week until reaching 5 mg (or hydrocortisone 20 mg) is suggested. After tapering daily prednisolone to 5 mg (or hydrocortisone 20 mg), GI-AI differentiation methods include measuring early morning serum cortisol 24 hours after the last steroid administration. If the level is between 3.6-12.7 μg/dL, or if the 30-minute cortisol level in a 250 μg ACTH stimulation test is between 12.7-20 μg/dL and the 60-minute cortisol level is between 13.8-18.1 μg/dL, it is considered GI-AI. Continuous steroid administration (hydrocortisone 15 mg AM + 5 mg early PM) is then given, and after 2-3 weeks, the adrenal insufficiency test is performed again. Tapering is recommended once the test results have normalized [ 19].
CONCLUSION
The intricate relationship between the brain’s hormonal regulatory structures, such as the hypothalamus and pituitary gland, and CIRCI in patients with neurological disorders highlights the importance of recognizing and managing this complex clinical scenario. Neurological conditions may predispose patients to CIRCI, necessitating early identification and intervention. Additionally, the frequent use of steroids in the treatment of these disorders may lead to steroid insufficiency, further complicating patient care.
Physicians must remain vigilant to these interactions and strive to effectively evaluate and manage patients with CIRCI and steroid insufficiency in the context of neuro-critical care. This involves prompt diagnosis, judicious steroid administration, and diligent monitoring for potential adverse effects. A comprehensive understanding of the interplay between neurological disease, CIRCI, and steroid insufficiency is essential for optimizing patient care and improving outcomes in this challenging patient population. By fostering awareness and enhancing the knowledge base of neuro-intensivists, we can ultimately contribute to the advancement of patient-centered care for those with neuro-critical disease and associated endocrine complications.
Acknowledgements
This research was supported by a grant from the Korean Government Ministr y of Science and ICT (MSIT) (2022R1A2C2011941), and Asan Institute for Life Sciences, Asan Medical Center - 2023IP0040, 2023IP0037, 2023IP0111 (Seoul, Republic of Korea), and Digital Healthcare Research Grant through the Seokchun Caritas Foundation (SCY2107P).
Fig. 1.
Hypothalamic-pituitary-adrenal (HPA) axis regulation. This schematic diagram illustrates the key components and interactions within the HPA axis, highlighting the regulatory role of the hypothalamus, pituitary gland, and adrenal cortex in stress response and hormonal balance. The figure demonstrates the release of corticotropin-releasing hormone (CRH) from the hypothalamus, which triggers the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland. Subsequently, ACTH stimulates the adrenal cortex to produce cortisol, the primary stress hormone. The HPA axis is regulated through a negative feedback loop, where increased cortisol levels inhibit the release of both CRH and ACTH, maintaining hormonal homeostasis. 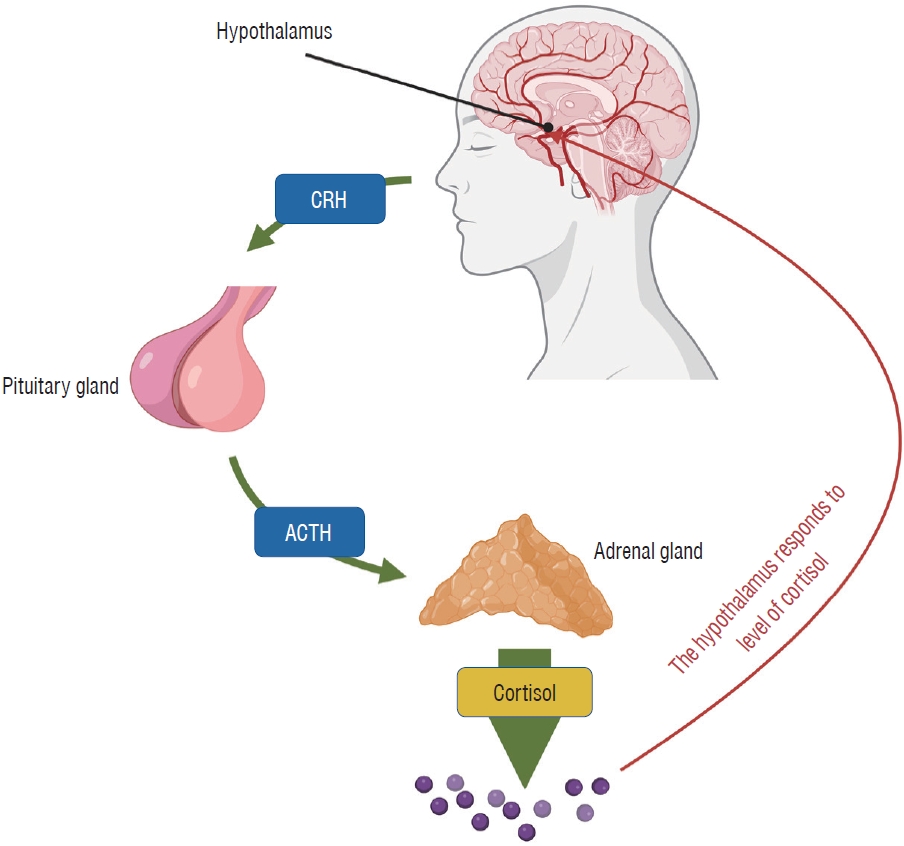
Fig. 2.
Steroidogenesis in the adrenal gland. This schematic diagram provides an overview of the key enzymatic processes involved in steroidogenesis within the adrenal gland, focusing on the synthesis of mineralocorticoids, glucocorticoids, and adrenal androgens. The figure depicts the conversion of cholesterol to pregnenolone, serving as the initial and rate-limiting step in steroidogenesis. It further illustrates the specific enzymatic pathways and intermediate compounds leading to the production of aldosterone (mineralocorticoid), cortisol (glucocorticoid), and dehydroepiandrosterone (DHEA; adrenal androgen). Additionally, the figure emphasizes the distinct functional zones of the adrenal cortex, highlighting the zona glomerulosa (mineralocorticoid production), zona fasciculata (glucocorticoid production), and zona reticularis (adrenal androgen production). Adopted from Arlt and Stewart [ 3] with permission. 3 ß HSD : 3-beta (ß)-hydroxysteroid dehydrogenase (HSD), P450c21 : human cytochrome P450c21 (steroid 21-hydroxylase, CYP21A2), CYP11B2 : aldosterone synthase (previously known as corticosterone methyloxidase), P450c17 : steroid 17 alphahydroxylase/17,20 lyase, CYP11B : steroid 11β-hydroxylase, SULT2A1 : dehydroepiandrosterone sulfotransferase. 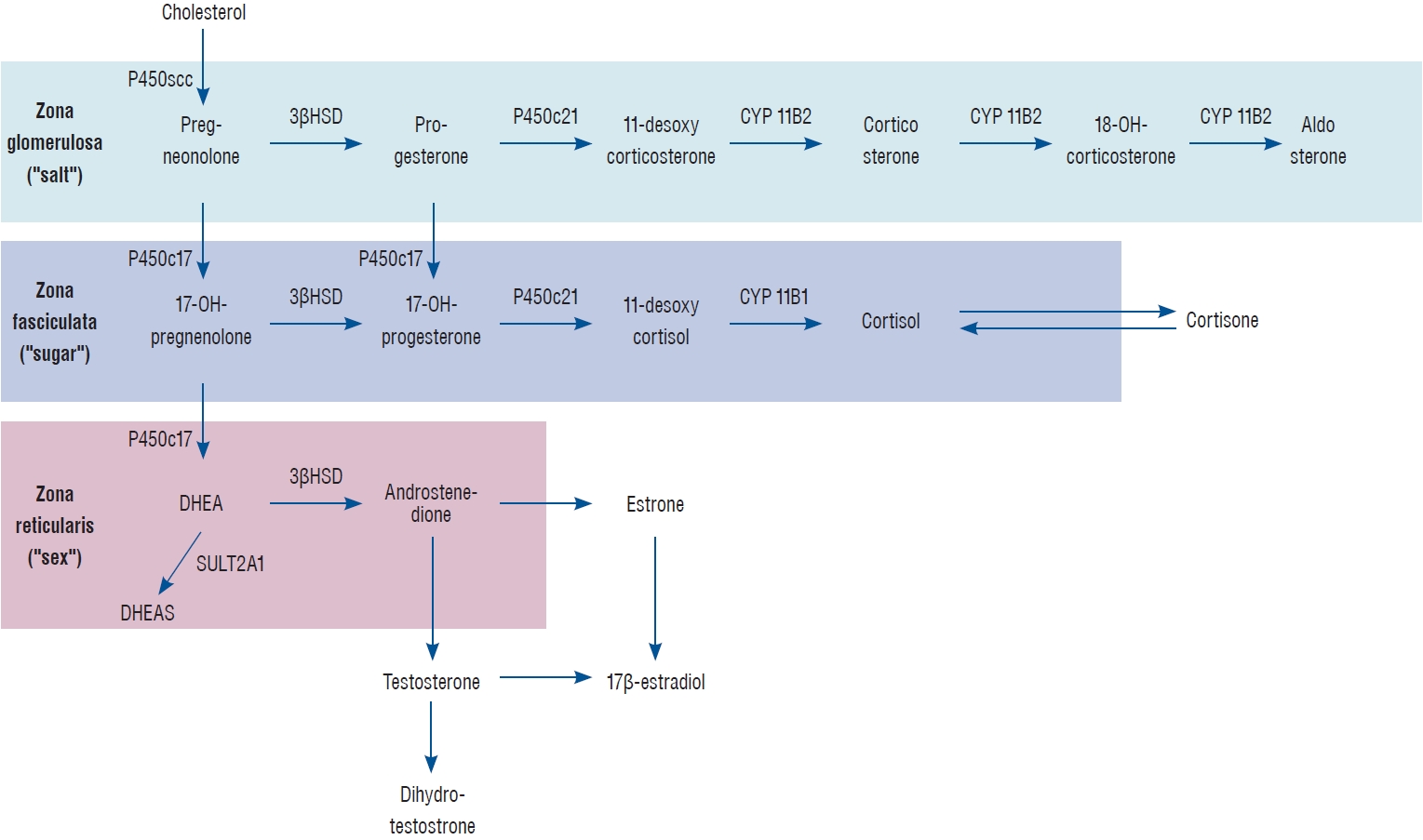
Fig. 3.
. Glucocorticoid synthesis at rest and stress [ 4]. This graphical representation compares the rate and regulation of glucocorticoid synthesis, specifically cortisol, under resting conditions and during stress. The figure highlights the role of the hypothalamic-pituitary-adrenal (HPA) axis in modulating cortisol production in response to various stressors. Under resting conditions, the HPA axis maintains basal cortisol levels through a tightly regulated negative feedback loop. In contrast, during stress, the HPA axis is activated, resulting in increased secretion of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), ultimately leading to elevated cortisol production by the adrenal cortex. The figure emphasizes the crucial role of cortisol in mediating physiological adaptations to stress, such as mobilizing energy reserves and modulating the immune response. Adopted from Annane et al. [ 1] with permission. AVP : arginine vasopressin, TLR : Toll-like receptor, ANS : autonomic nervous system, DAMPs : damage-associated molecular patterns, PAMPs : pathogen-associated molecular patterns. 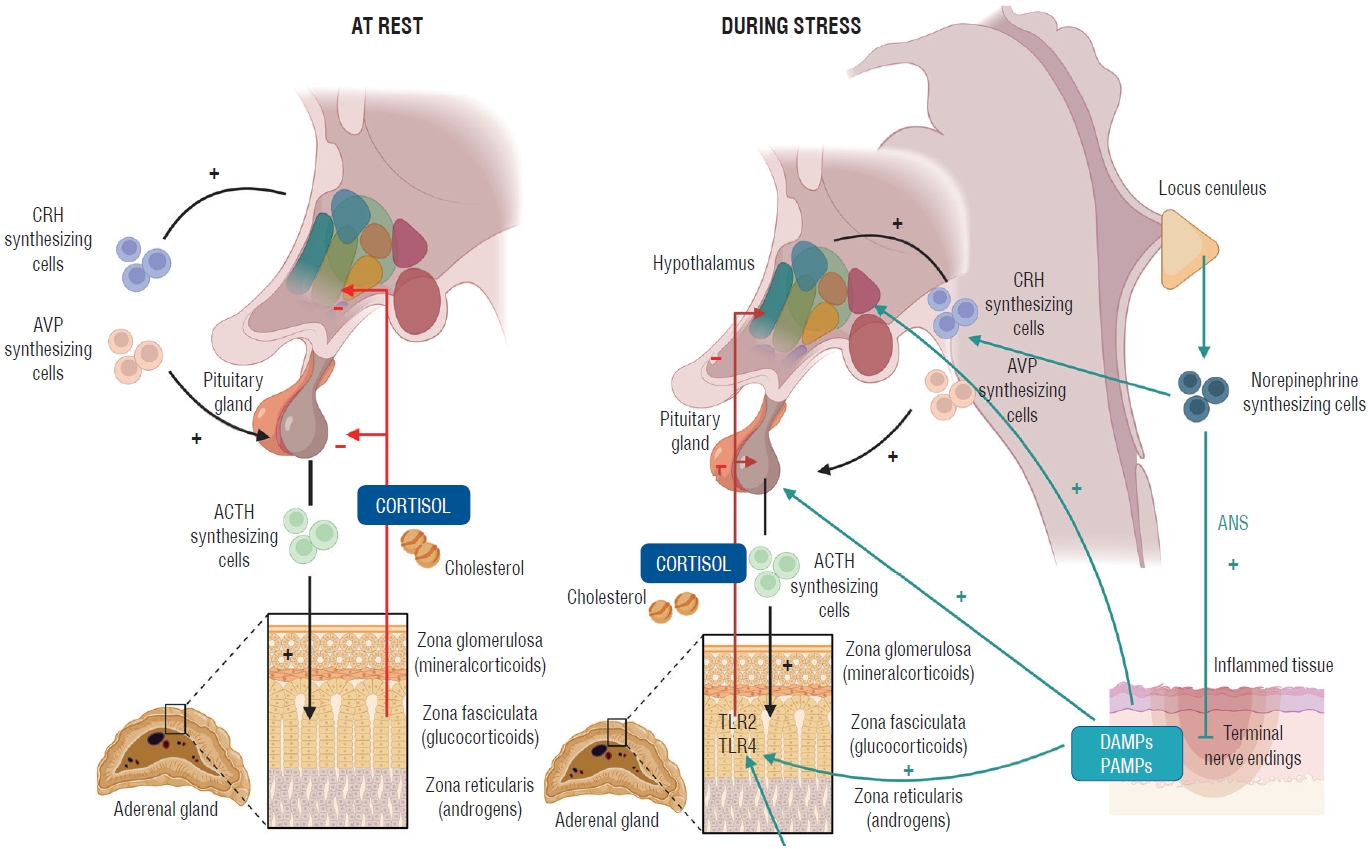
Fig. 4.
Activity of the hypothalamic-pituitary-adrenal (HPA) axis under different conditions [ 7]. This three-panel figure illustrates the activity of the HPA axis under normal conditions (A), during an appropriate response to stress (B), and during an inappropriate response to critical illness (C). (A) demonstrates the basal activity of the HPA axis under normal conditions, with the hypothalamus releasing corticotropin-releasing hormone (CRH), stimulating the anterior pituitary to secrete adrenocorticotropic hormone (ACTH), which in turn triggers the adrenal cortex to produce cortisol. A negative feedback loop maintains hormonal homeostasis. (B) shows an appropriate response to stress, where the HPA axis is activated, resulting in increased secretion of CRH and ACTH, and subsequently, elevated cortisol production. This adaptive response enables the body to cope with the stressor by mobilizing energy reserves, modulating immune response, and maintaining homeostasis. (C) depicts an inappropriate response to critical illness, characterized by a blunted or dysregulated HPA axis. This may lead to inadequate cortisol production, contributing to poor outcomes such as persistent inflammation, immune dysregulation, and multi-organ dysfunction. This panel highlights the potential need for exogenous glucocorticoid administration to counteract the detrimental effects of HPA axis dysfunction during critical illness. Adopted from Cooper and Stewart [ 8] with permission. HIV : human immunodeficiency virus. 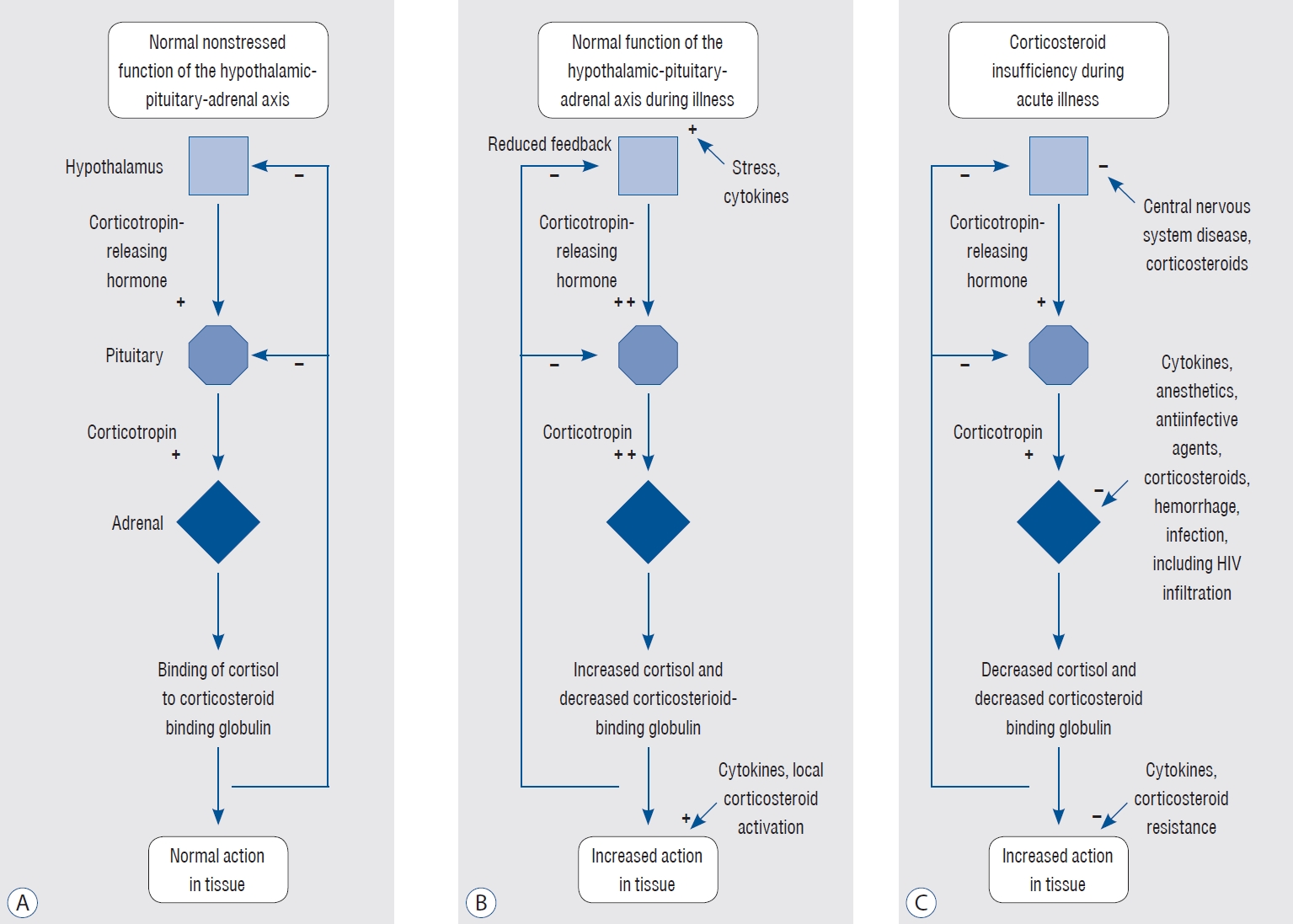
Fig. 5.
Pathophysiology of glucocorticoid induced hypothalamic-pituitary-adrenal (HPA) axis suppression [ 5]. This schematic diagram illustrates the pathophysiological mechanisms underlying glucocorticoid-induced HPA axis suppression, focusing on the negative feedback loop triggered by exogenous glucocorticoid administration. The figure demonstrates that exogenous glucocorticoids, when administered in pharmacological doses, result in elevated systemic cortisol levels. This increase in cortisol suppresses the production and release of corticotropin-releasing hormone (CRH) from the hypothalamus and adrenocorticotropic hormone (ACTH) from the anterior pituitary gland, effectively inhibiting the normal activity of the HPA axis. Consequently, the adrenal cortex produces lower amounts of endogenous cortisol, leading to adrenal insufficiency and a reduced ability to respond to stressors. The figure highlights the importance of recognizing and managing glucocorticoid-induced HPA axis suppression to prevent complications, such as adrenal crisis, during withdrawal or rapid tapering of exogenous glucocorticoids. Adopted from Prete and Bancos [ 19] with permission. GC : glucocorticoid, IV : intravenous, IM : intramuscular. 
Table 1.
Characteristics, dosage comparisons, and clinical applications of systemic corticosteroids in relation to hydrocortisone [ 3]
|
Approximate equivalent dose (mg) |
Relative glucocorticoid activity |
Relative mineralocorticoid activity |
Duration of action (hours) |
General therapeutic indication |
|
Glucocorticoids |
|
|
|
|
|
|
Short-acting |
|
|
|
|
|
|
|
Hydrocortisone |
20 |
1 |
1 |
8-12 |
Relatively high mineralocorticoid activity makes it suitable for use in adrenal insufficiency |
|
|
Cortisone |
25 |
0.8 |
0.8 |
8-12 |
Similar to hydrocortisone |
|
Intermediate-acting |
|
|
|
|
|
|
|
Prednisone |
5 |
4 |
0.8 |
12-36 |
High glucocorticoid activity makes it useful for long-term treatment, and as an anti-inflammatory/immunosuppressant |
|
|
Prednisolone |
5 |
4 |
0.8 |
12-36 |
Similar to prednisone |
|
|
Methylprednisolone |
4 |
5 |
Minimal |
12-36 |
Anti-inflammatory/immunosuppressant |
|
|
Triamcinolone |
4 |
5 |
0 |
12-36 |
Anti-inflammatory/immunosuppressant |
|
Long-acting |
|
|
|
|
|
|
|
Dexamethasone |
0.75 |
30 |
Minimal |
36-72 |
Anti-inflammatory/immunosuppressant; used especially when water retention is undesirable given its minimal mineralocorticoid activity |
|
Usually reserved for short-term use in severe, acute conditions given its high potency and long-duration of action |
|
|
Betamethasone |
0.6 |
30 |
Negligible |
36-72 |
Similar to dexamethasone |
|
Mineralocorticoids |
|
|
|
|
|
|
Fludrocortisone |
0 |
10-15 |
125-150 |
12-36 |
Used for aldosterone replacement |
Table 2.
Frequently administered systemic glucocorticoids, in comparison to prednisolone 5 mg
|
Drug type |
Drug |
Prednisolone equivalent doses, using 5 mg prednisolone as reference (mg) |
|
Short acting glucocorticoids with lower potency (biological half-life <12 hours) |
Hydrocortisone |
20 |
|
Cortisone acetate |
25 |
|
Deflazacort |
6 |
|
Intermediate acting glucocorticoids with intermediate potency (biological half-life 12-36 hours) |
Prednisone |
5 |
|
Prednisolone |
5 |
|
Methylprednisolone |
4 |
|
Triamcinolone |
4 |
|
Long-acting glucocorticoids with higher potency (biological half-life 36-54 hours) |
Dexamethasone |
0.50 |
|
Betamethasone |
0.50 |
Table 3.
Main mechanisms of critical illness-related corticosteroid insufficiency
|
General defect |
Main mechanism |
Key factor |
|
Decrease in cortisol production |
|
Altered adrenal synthesis of cortisol |
Necrosis/hemorrhage |
Acute kidney failure; hypo‑coagulation; disseminated intravascular coagulation; cardiovascular collapse; tyrosine kinase inhibitors |
|
Decreased availability of esterified cholesterol |
Depletion in adrenal storage regulated by annexin A1—formyl peptide receptors downregulated scavenger receptor‑B1 |
|
Inhibition of steroidogenesis |
Immune cells/toll‑like receptors/cytokines |
|
Drugs e.g., sedatives, corticosteroids |
|
ACTH‑like molecules e.g., corticostatins |
|
Altered synthesis of CRH/ACTH |
Necrosis/hemorrhage |
Cardiovascular collapse; disseminated intravascular coagulation; treatment with vasopressor agents |
|
Inhibition of ACTH synthesis |
Glial cells/nitric oxide mediated neuronal apoptosis |
|
Increased negative feedback from circulating cortisol following up‑regulation of ACTH‑independent mechanisms of cortisol synthesis |
|
Drugs e.g., sedatives, anti‑infective, psychoactive agents |
|
Inappropriate cessation of glucocorticoid treatment |
|
Alteration of cortisol metabolism |
Decreased cortisol transport |
Downregulation of liver synthesis of cortisol‑binding globulins and albumin |
|
Reduced cortisol breakdown |
Decreased expression and activity of the glucocorticoid‑inactivating 5‑reductase enzymes in the liver with putative role of bile acids; decreased expression and activity of the hydroxysteroid dehydrogenase in the kidney |
|
Tissue resistance to cortisol |
Inadequate GR-α activity |
Multifactorial etiology including reduced GR‑α density and transcription and excessive NF-κB activation |
Table 4.
|
Clinical symptom |
|
General |
Fever, asthenia |
|
Neurological |
Confusion |
|
Delirium coma |
|
Cardiovascular |
Hypotension refractory to fluid resuscitation decreased sensitivity to catecholamines high cardiac index |
|
Digestive |
Nausea vomiting |
|
Intolerance to enteral nutrition |
|
Respiratory |
Persistent hypoxia |
|
Laboratory |
Hypoglycemia |
|
Hyponatremia |
|
Hyperkalemia metabolic acidosis hypereosinophilia |
|
Imaging |
Hemorrhage or necrosis in hypothalamus, pituitary gland or adrenal gland |
References
1. Annane D, Pastores SM, Arlt W, Balk RA, Beishuizen A, Briegel J, et al : Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a multispecialty task force of the society of critical care medicine (SCCM) and the european society of intensive care medicine (ESICM). Intensive Care Med 43 : 1781-1792, 2017    2. Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al : Guidelines for the diagnosis and management of critical illnessrelated corticosteroid insufficiency (CIRCI) in critically ill patients (part I): society of critical care medicine (SCCM) and european society of intensive care medicine (ESICM) 2017. Intensive Care Med 43 : 1751-1763, 2017    3. Arlt W, Stewart PM : Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am 34 : 293-313, viii, 2005   4. Bendel S, Koivisto T, Ruokonen E, Rinne J, Romppanen J, Vauhkonen I, et al : Pituitary-adrenal function in patients with acute subarachnoid haemorrhage: a prospective cohort study. Crit Care 12 : R126, 2008    5. Broersen LH, Pereira AM, Jørgensen JO, Dekkers OM : Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab 100 : 2171-2180, 2015    6. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al : Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 80 : 6-15, 2017    7. Cohan P, Wang C, McArthur DL, Cook SW, Dusick JR, Armin B, et al : Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med 33 : 2358-2366, 2005   8. Cooper MS, Stewart PM : Corticosteroid insufficiency in acutely ill patients. N Engl J Med 348 : 727-734, 2003   9. Dimopoulou I, Tsagarakis S, Kouyialis AT, Roussou P, Assithianakis G, Christoforaki M, et al : Hypothalamic-pituitary-adrenal axis dysfunction in critically ill patients with traumatic brain injury: incidence, pathophysiology, and relationship to vasopressor dependence and peripheral interleukin-6 levels. Crit Care Med 32 : 404-408, 2004   10. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al : Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47 : 1181-1247, 2021   11. Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, et al : 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 53 : e282-e361, 2022   12. Joseph RM, Hunter AL, Ray DW, Dixon WG : Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum 46 : 133-141, 2016    13. Karamouzis I, Pagano L, Prodam F, Mele C, Zavattaro M, Busti A, et al : Clinical and diagnostic approach to patients with hypopituitarism due to traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and ischemic stroke (IS). Endocrine 52 : 441-450, 2016    14. Laugesen K, Broersen LHA, Hansen SB, Dekkers OM, Sørensen HT, Jorgensen JOL : Management of endocrine disease: glucocorticoid-induced adrenal insufficiency: replace while we wait for evidence? Eur J Endocrinol 184 : R111-R122, 2021   16. Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al : Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 36 : 1937-1949, 2008   17. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al : Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50 : e344-e418, 2019   18. Powner DJ, Boccalandro C : Adrenal insufficiency following traumatic brain injury in adults. Curr Opin Crit Care 14 : 163-166, 2008   19. Prete A, Bancos I : Glucocorticoid induced adrenal insufficiency. BMJ 374 : n1380, 2021   20. Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A : Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA 298 : 1429-1438, 2007   21. Tanriverdi F, De Bellis A, Bizzarro A, Sinisi AA, Bellastella G, Pane E, et al : Antipituitary antibodies after traumatic brain injury: is head traumainduced pituitary dysfunction associated with autoimmunity? Eur J Endocrinol 159 : 7-13, 2008   22. Tanriverdi F, De Bellis A, Ulutabanca H, Bizzarro A, Sinisi AA, Bellastella G, et al : A five year prospective investigation of anterior pituitary function after traumatic brain injury: is hypopituitarism long-term after head trauma associated with autoimmunity? J Neurotrauma 30 : 1426-1433, 2013   23. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al : Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 8 : 267-276, 2020   24. Weant KA, Sasaki-Adams D, Dziedzic K, Ewend M : Acute relative adrenal insufficiency after aneurysmal subarachnoid hemorrhage. Neurosurgery 63 : 645-649; discussion 649-650, 2008    25. Wijdicks EF, Findlay JY, Freeman WD, Ayan Sen M : Mayo clinic critical and neurocritical care board review. Oxford : Oxford University Press, 2019
|
|





















