Choi and Lee: Cervical Myelopathy Induced by Posterior Vertebral Body Osteolysis after Cervical Disc Arthroplasty
Abstract
Cervical disc arthroplasty (CDA) has become more widespread and diverges from the conventional technique used in anterior cervical fusion for cervical degenerative disc disease. As arthroplasty has become a popular treatment option, few complications have been reported in the literature. These include subsidence, expulsion, posterior avulsion fractures, heterotopic ossification, and osteolysis. One of the critical complications is osteolysis, but current studies on this subject are limited in terms of not elucidating the incidence, etiology, and consequences. The authors present two cases, who presented with clinical signs of gradually worsening myelopathy induced by posterior vertebral body osteolysis, 2 years after CDA. Subsequently, the patient underwent posterior decompression and fusion without prosthesis removal. Postoperatively, the clinical symptoms gradually resolved, with no severe deficits. The present rare cases highlight the osteolysis that occurs after CDA, which can cause cervical myelopathy, and suggest spine surgeons to be alert to this fatal complication.
Key Words: Arthroplasty В· Cervical vertebrae В· Myelopathy В· Osteolysis.
INTRODUCTION
Cervical disc arthroplasty (CDA) is a viable and effective treatment for cervical degenerative disc diseases and has been proposed as an alternative to conventional anterior cervical discectomy and fusion [ 3, 7]. Progressive vertebral body osteolysis is considered one of the major complications after CDA and presents various clinical features, ranging from asymptomatic bone loss to severe bone loss, which might lead to acquired deformity and neurological deterioration requiring revision surgery [ 10, 14]. While the majority of studies have focused on the identifications of etiology, risk factors of osteolysis as well as their feasible prevention measures, the question that might be a crucial concern to all surgeons whether the presence of osteolysis would pose significant impact on the clinical outcomes has not been properly addressed currently. We present two cases of progressive osteolysis of the vertebral body in the superior and inferior keel of the alloy endplate, leading to severe myelopathy. The clinical course, sequential diagnostic radiographic images, and surgical treatment were reviewed in detail.
CASE REPORT
Surgical procedure
A standard Smith-Robinson approach to ventrally access the pathological cervical level using a transverse incision was performed for every patient [ 13]. Full resection of the anterior longitudinal ligaments, full decompression of the intradiscal space, and complete resection of the posterior longitudinal ligament were performed before insertion of the artificial disc implants. Care was taken not to manipulate the bilateral uncinate process and to minimize disruption to the bilateral longus colli muscles. For the implants inserted during the CDA procedures, ROTAIO (SIGNUS Medizintechnik GmbH, Alzenau, Germany) cervical artificial disc products were used. In both cases, after several trials before actual insertion of the real implants, a proper device parameter of 5-mm tall with 17 mm width and 15 mm depth that matched the size of the end plates of each patient was chosen. The operating surgeon (corresponding author) confirmed the stable anchoring of the device after insertion but did not choose a device that would increase the new disc height by >2 mm compared with the original disc height. The midpoint between the bilateral longus colli muscles and the spinous process, which was visualized on an intraoperative fluoroscopy image, was used as the вҖңmidlineвҖқ marker during insertion of the device at each level. Both the superior and inferior plates of each device were inserted in depth to reach as close to the posterior margin of the end plates at the corresponding level of the cervical vertebral body as possible under fluoroscopic guidance so that the core of the device could be located rear to the midpoint of the anterior-posterior length of the intervertebral disc or end plates.
Case 1
A 64-year-old male presented with clinical signs of neck pain and right C5 and C6 radicular arm pain over the past few years. Neurological examination revealed weakness of the right deltoid and biceps with 4/5 strength. Magnetic resonance imaging (MRI) showed a herniated disc to the right at the C4-5 level causing severe neural foraminal stenosis and stenosis with cord compression at the C5-6 level ( Fig. 1A- C). Patients elected to proceed with CDA. The Smith-Robinson technique was used to approach the C4-5-6 disc spaces. The patient was discharged on the second post-operative day with complete resolution of radicular pain and improvement in deltoid weakness. Computed tomography (CT) was performed immediately after surgery ( Fig. 1D and E). Over the course of 2 years, the patient experienced complete pain relief. However, the patient reported a steady increase in axial neck pain and gait disturbance. A CT scan, MRI, plain radiographs were obtained 25 months after surgery for the evaluation of his neck pain and myelopathic symptom ( Fig. 2A- C). A lucency was seen surrounding the posterior margin of the endplates at the C4-5-6 levels. In addition, myelopathy with signal changes was observed. This radiographic study showed severe osteolysis surrounding the artificial implant, extending to the superior and inferior aspects of the endplates as well as to the posterior wall of the vertebral body. The cause of severe osteolysis prompted concern for the stability of the artificial implant as well as inflammatory sequelae with/without infection. Laboratory tests revealed a normal range of C-reactive protein (<0.3 mg/dL), erythrocyte sedimentation rate (<10 mm/hr), and white blood cell count (<8000 cells/mm 3) without eosinophilia. Prompt surgical intervention was performed for extended decompression and posterior fusion. Considering the severe compressive lesions, wide posterior decompression ranging from C4 to C7 and posterior screw fixation ranging from C4 to T1 were performed. After revision surgery, the patient showed an improvement in neck pain and gait disturbance. The patient showed resolution of the osteolytic process and stable arthrodesis at 12 months on plain radiographs after revision surgery ( Fig. 2D).
Case 2
A 44-year-old male patient was referred to the outpatient department for surgical treatment of his cervical condition after having suffered from radicular arm pain over the past 2 years. He was barely spared from left arm radiculopathy. MRI showed severe stenosis of the left neural foramen at C5-6 level ( Fig. 3A). As in case 1, given the operational demands within his field, the CDA was performed at the C5-6 disc space. After the surgical procedure, the patient was discharged from the hospital on postoperative day. While symptoms such as a tingling sensation on the left fingertips persisted, he was satisfied with the postoperative result. The postoperative plain radiographs showed an appropriately located artificial disc ( Fig. 3B and C). The patient was in fair condition, both clinically and radiologically, up to a couple of years after the initial procedure. However, the patient reported gait disturbance, sudden electricity, and clumsiness in both outpatient clinics. A CT scan and MRI were obtained 26 months after surgery for the evaluation of his arm pain and myelopathic symptoms ( Fig. 4A and B). Lucency was observed surrounding the posterior margin of the endplates at the C6 level, and myelopathy with signal change was observed, as in case 1. There were no abnormal spinal motions, including artificial implants, and no increase in inflammatory markers in the laboratory tests. He required an additional intervention, laminectomy and posterior fusion of C5-6 ( Fig. 4C and D). After the procedure, the patient was discharged a few days later, leaving numbness in both fingers.
DISCUSSION
This unprecedented inflammatory deposition back to the device and into the spinal canal eventually incurring the clinical cervical spondylotic myelopathy neurologic feature might not have been discovered or anticipated either by the surgeon or the patient themselves, although they have been followed up for years with regular functional radiographs after the index procedure. Given the years of use of ROTAIO and the scarcity of complications, the safety of this implant has been suggested. Therefore, the authors were confounded by this case and sought an explanation for this phenomenon.
Among the few scattered but relevant references, Wenger and Markwalder [ 15] have warned spine surgeons of unexpected heterotopic ossification (HO) associated with myelopathy following cervical disc prosthesis implantation. The conventional explanatory hypothesis for this untoward phenomenon is that the permanent micro stress between the bony endplate and the device, which might have originated from the nonphysiological prosthesis motion, might have promoted osseous spur formation and its enlargement [ 1]. An untoward synergy between the non-physiological motions created from the ventrally placed prosthesis with a relatively smaller caliber, along with the presumably insufficiently resected remnant dorsal osteophytes, might have triggered this appositional bone growth over the residual osteophytes, whose growth is well known to be promoted by the untoward segmental motion. However, osteolysis after CDA appears to be a more serious complication than HO deposition on the prosthesis. The incidence of these cases was reported to be 8% to 63.7% (asymptomatic) [ 10, 11]. Based on the literature, a pattern within 1 year in which osteolysis is the majority, and a delayed pattern beyond 1 year is rare [ 10- 12]. By contrast, Hacker et al. [ 6] reported an incidence of 4.2% for symptomatic osteolysis, which occurred up to 4 years after CDA. It is controversial whether osteolysis is related to polyethylene and stress shielding of implants. Early osteolysis within 1 year may be due to insufficient vascular supplement, and later osteolysis may be related to mechanical stress shielding underlying vascular comprimise [ 9]. From the two cases in our review, symptomatic osteolysis was concordantly diagnosed after 2 years of follow-up, suggesting that this inflammatory process could present as a clinical feature even in remote years after the index surgery. The characteristics of the pattern of osteolysis within the vertebral body in these cases need to be reconciled. The blooming pattern of the lucency was observed both above and below each implantвҖҷs upper and lower plates, and this blooming pattern of osteolysis was posteriorly located, whereas the anterior aspect of the vertebral body appeared largely unaffected. The previously reported potential cause of osteolysis is an immune-mediated process that may result in a local inflammatory reaction leading to hastened bone resorption with a continuous inflammatory zone adjacent to the prosthesis, where macrophage exhaustion, reactive oxygen intermediates, and inflammatory cytokines induce soft tissue damage and inflammation [ 4, 9]. Although there are no studies on metal sensitivity related to CDA, a meta-analysis evaluating subjects who had metal sensitivity tests on total joint replacement reported a higher probability of metal allergy and a higher risk of implant failures [ 5]. Another speculated cause is infection, which is a concern when bone resorption is evident. For example, Cutibacterium acnes, which can present with no evident signs of infection [ 8]. In these cases, although the inflammatory markers indicated normal values, it was difficult to determine whether there was an infection because bone culture could not be performed during the revision surgery. Finally, bone loss related to the distribution of the loading force can be considered. A large shell angle on the anterior aspect of the vertebral body is associated with bone loss [ 2]. A larger angle seems to converge most of the loading force posteriorly, resulting in bone loss [ 2]. However, there could be no clear elucidation, especially regarding its isolation from the rear to the initial implant, sparing the preferential ventral aspect of the addressed cervical level, which would considerably contribute and subsequently provide the key to developing a validated diagnostic measure for this implant-related osteolysis. A conversion to solid anterior fusion after full removal of the causative mobile device, as well as the inflammatory deposits, would be the preferred revision measure over simple posterior decompression and fixation for the sake of cervical curvature maintenance. Moreover, this presumably consolidated, functionally deprived prosthesis after secondary posterior fixation surgery might not be fully immobilized, retaining its inherent mobility to be f lexed. This post-surgical phenomenon suggests the lack of capability for posterior stand-alone fixation to provide firm stability over the functionally deprived cervical motional segment after untoward CDA insertion. Despite this skepticism, the authors have prioritized the extent of signal change inside the cervical spinal cord and consequent expected levels of decompression, which might only be guaranteed through full multilevel, extensive laminectomy instead of regional decompression through the interbody spaces during the revisional anterior approach. Fortunately, owing to its chemical and inflammatory nature, the loculated collections expected to be spread onto the ventral aspect of the cervical spinal cord over the extent of a couple of cervical segments spontaneously regressed after this indirect posterior decompression without any manipulation.
CONCLUSION
There is a paucity in the past literature providing a proper interpretation of this unexpected, extensive osteolysis-related inflammatory deposition to a functioning cervical artificial disc prosthesis incurring late myelopathy. Mechanical, infectious, and immune-mediated osteolytic processes might explain the clinical symptoms and radiographic progression of osteolysis. Conversion to solid posterior fusion with full decompression has provided viable clinical recuperation even without the removal of the causative mobile device.
Fig.В 1.
Pre-operative T2-weighted magnetic resonance imaging with definite evidence of foraminal stenosis and herniated intervertebral disc. Sagittal image (A) and axial image at C4-5 (B) and C5-6 (C). The three-dimensional reconstructed coronal (D) and sagittal (E) scans by cervical spine computed tomography obtained immediately after surgery. This view shows no lucency and bony destruction around the prosthesis. 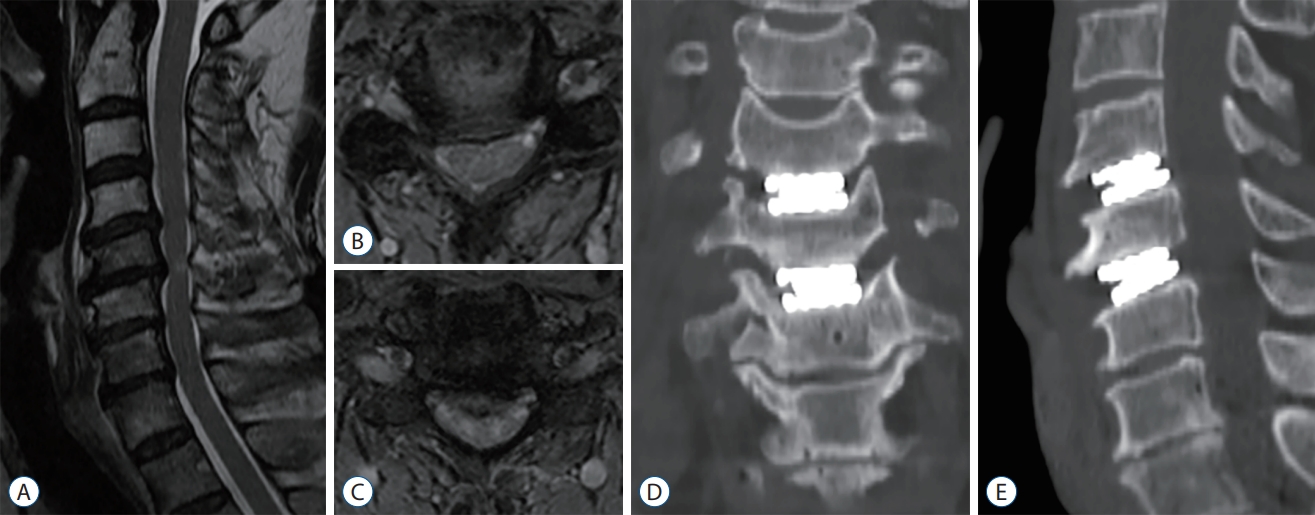
Fig.В 2.
Magnetic resonance imaging sagittal, axial (A and B) and computed tomography (C) scans obtained 25 months after surgery. A lucency was seen surrounding the posterior margin of the endplates at the C4-5-6 levels (arrows). Red dotted line showed cord signal change. Plain radiographs. Lateral (D) views of cervical spine at 12 months after revision surgery showing stoppage of the osteolytic process and stable structure. 
Fig.В 3.
Pre-operative axial image of magnetic resonance imaging (A). Plain radiographs. Anteroposterior (B) and lateral (C) views of cervical spine immediately after surgery. These views show no lucency and bony destruction around the prosthesis. 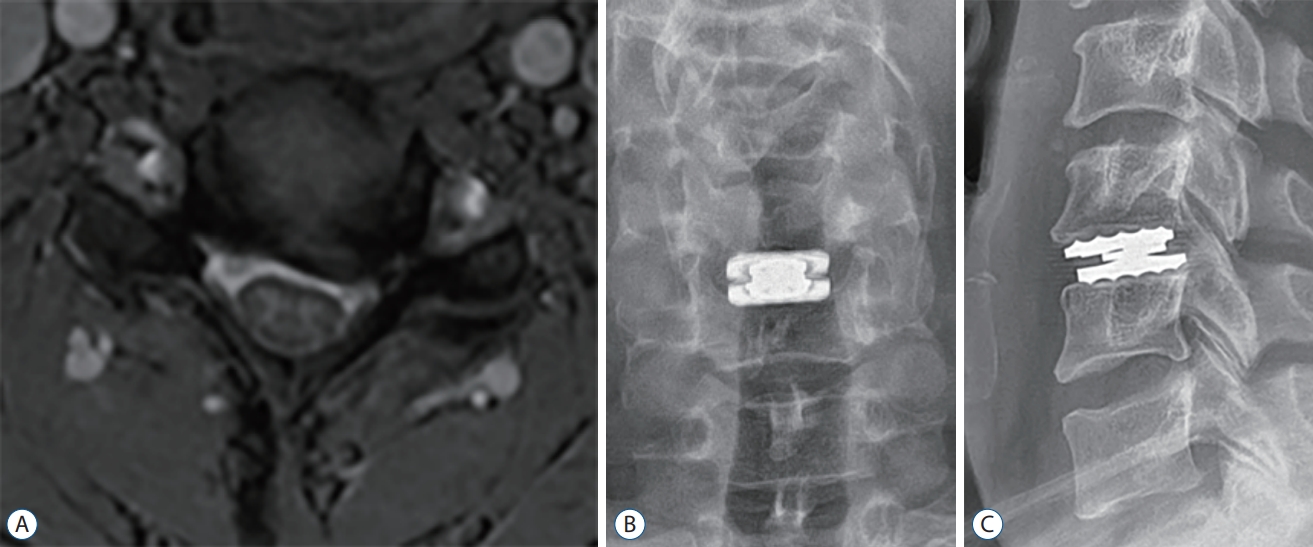
Fig.В 4.
Magnetic resonance imaging (MRI) sagittal (A) and computed tomography (B) scans obtained 26 months after surgery. A lucency was seen surrounding the posterior margin of the endplates at the C5-6 level (arrow). Plain radiograph lateral (C) and MRI sagittal (D) views of cervical spine immediately after revision surgery. 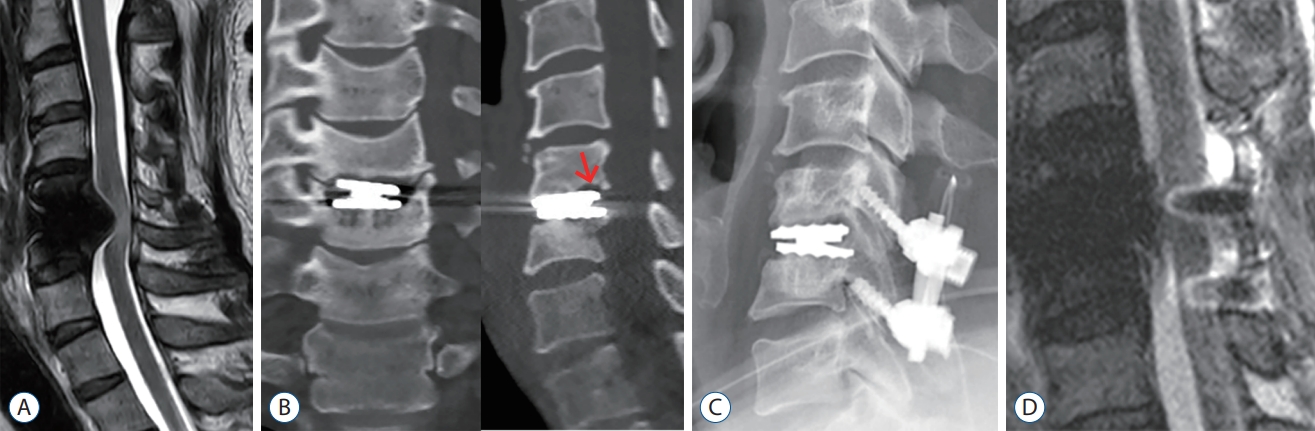
References
1. Chang PY, Wu JC, Mayo BC, Massel DH, Wang MY : Heterotopic ossification in cervical disc arthroplasty. Contemp Spine Surg 18 : 1-5, 2017  2. Chen TY, Chen WH, Tzeng CY, Huang CW, Yang CC, Chen HT, et al : Anterior bone loss after cervical Bryan disc arthroplasty: insight into the biomechanics following total disc replacement. Spine J 20 : 1211-1218, 2020   3. Gao F, Mao T, Sun W, Guo W, Wang Y, Li Z, et al : An updated meta-analysis comparing artificial cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc disease (CDDD). Spine (Phila Pa 1976) 40 : 1816-1823, 2015   4. Gawkrodger DJ : Metal sensitivities and orthopaedic implants revisited: the potential for metal allergy with the new metal-on-metal joint prostheses. Br J Dermatol 148 : 1089-1093, 2003   5. Granchi D, Cenni E, Giunti A, Baldini N : Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br 94 : 1126-1134, 2012  6. Hacker FM, Babcock RM, Hacker RJ : Very late complications of cervical arthroplasty: results of 2 controlled randomized prospective studies from a single investigator site. Spine (Phila Pa 1976) 38 : 2223-2226, 2013  7. Hisey MS, Zigler JE, Jackson R, Nunley PD, Bae HW, Kim KD, et al : Prospective, randomized comparison of one-level mobi-C cervical total disc replacement vs. anterior cervical discectomy and fusion: results at 5-year follow-up. Int J Spine Surg 10 : 10, 2016    8. Hsu JE, Bumgarner RE, Matsen FA 3rd : Propionibacterium in shoulder arthroplasty: what we think we know today. J Bone Joint Surg Am 98 : 597-606, 2016  9. Joaquim AF, Lee NJ, Lehman RA Jr, TumialГЎn LM, Riew KD : Osteolysis after cervical disc arthroplasty. Eur Spine J 29 : 2723-2733, 2020    10. Kieser DC, Cawley DT, Fujishiro T, Mazas S, BoissiГЁre L, Obeid I, et al : Risk factors for anterior bone loss in cervical disc arthroplasty. J Neurosurg Spine 29 : 123-129, 2018   12. Kim SH, Chung YS, Ropper AE, Min KH, Ahn TK, Won KS, et al : Bone loss of the superior adjacent vertebral body immediately posterior to the anterior flange of Bryan cervical disc. Eur Spine J 24 : 2872-2879, 2015    13. Smith GW, Robinson RA : The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 40-A : 607-624, 1958   14. TumialГЎn LM, Gluf WM : Progressive vertebral body osteolysis after cervical disc arthroplasty. Spine (Phila Pa 1976) 36 : E973-E978, 2011   15. Wenger M, Markwalder TM : Heterotopic ossification associated with myelopathy following cervical disc prosthesis implantation. J Clin Neurosci 26 : 154-156, 2016  
|
|




















