Siahaan, Tandean, Nainggolan, Tarigan, and Sitanggang: A Critical Analysis of Intracranial Hemorrhage as a Fatal Complication of Dengue Fever
Abstract
Dengue fever is the most rapidly spreading mosquito-borne virus in the world, infecting about 100 million individuals. A rare but possibly dangerous consequence of dengue illness is intracranial hemorrhage (ICH). Currently, the pathogenesis of ICH is unknown. A number of studies have found a variety of risk factors for ICH in dengue. In addition, studies have reported the use of emergency surgery while monitoring thrombocytopenia in the therapy of dengue ICH. This review enumerates the potential predictors of ICH in dengue, discusses the use of brain imaging, and mentions the possibility of emergency surgery.
Key Words: Intracranial haemorrhages ┬Ę Dengue ┬Ę Review.
INTRODUCTION
As the fastest-spreading mosquito-borne viral disease in the world, dengue fever (DF) affects over 100 million people and causes between 20000 and 25000 deaths annually in over 100 countries [ 38]. Dengue is an arboviral infection that is commonly transmitted by Aedes aegypti and, to a lesser extent, Aedes albopictus mosquitoes. It is hyperendemic in tropical and subtropical climates worldwide, and its incidence has increased in recent years, putting at risk nearly half of the worldŌĆÖs population [ 20]. The Dengue virus (DENV) is a flavivirus, which is an RNA virus with four antigenically distinct serotypes, namely the DENV 1-4 [ 18, 48]. The spectrum of clinical manifestations can range from infections with no discernible symptoms to a vast array of conditions with severe clinical manifestations. DF can range from mildly incapacitating to life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome in patients with DHF due to plasma leakage. Most likely, these conditions correspond to dynamically severe dengue infection spectrum phases [ 18]. The clinical phase of a DENV infection consists of the febrile, critical, and recovery phases. Plasma leakage shortly after the end of the febrile phase distinguishes DHF from DF, which bypasses the critical phase. Typically, plasma leakage in DHF is transient, lasting between 24 and 48 hours, and is intrinsically dynamic [ 20]. Neurological or central nervous system (CNS) involvement/complications of dengue are extremely rare, occurring less than one percent of the time [ 34]. Neurological manifestations of DHF are typically the result of multisystem dysfunction caused by liver failure, cerebral hypoperfusion, electrolyte imbalance, shock, cerebral edema, and hemorrhage caused by vascular leakage [ 13]. Intracranial hemorrhage (ICH) is one of the uncommon indications that dengue is affecting the CNS, primarily as a result of DF complications. Pathophysiology and treatment of ICH remain controversial. Vasculopathy, coagulopathy, platelet dysfunction, and thrombocytopenia are among the numerous proposed mechanisms. Dengue immunoglobulin (Ig) M, IgG, and non-structural protein 1 (NS1) antigen in the cerebrospinal fluid (CSF) of dengue patients suggested a breakdown of the blood-brain and blood-CSF barriers, as well as the development of vasculopathy due to immunopathological-related mechanisms [ 53]. Extremely few cases of ICH due to DENV infection have been documented [ 26]. Depending on the severity of the injury, it may be difficult to decide whether to treat this phenomenon conservatively or surgically given the limited number of documented cases and the lack of guidance [ 3]. The purpose of this research is to determine if there are any risk factors for intracranial bleeding in DF patients and, if so, when those risk factors should be considered and what kind of radiological imaging or surgical intervention should be performed if intracranial bleeding is suspected.
MATERIALS AND METHODS
Several scientific databases, such as PubMed and Science Direct, were consulted. The literature search employed the strategic Boolean term method of keywords relating to intracranial bleeding and DF synonyms. This reviewŌĆÖs primary inclusion criterion is an English study reporting ICH in DF. The only criterion for exclusion was a conservative approach. If a study reported a series of cases, the authors would exclude the conservative approach.
RESULTS
This study consisted of seven studies involving a total of 14 patients ( Fig. 1). Thirteen (93%) of the patients were male, while one (7%) was female. Thrombocytopenia was observed in every patient. Only one patient (7%) who mentioned the value of liver enzymes had elevated aspartate aminotransferase (AST)/alanine transaminase (ALT). All patients exhibited positive dengue serology; however, two studies involving a total of eight patients did not specify which serology was tested. Ten patients (71%) were found to have recovered from surgical intervention with platelet preservation, while four patients (29%) succumbed. Table 1 depicted patientsŌĆÖ characteristic.
DISCUSSION
The significance of early dengue IgG antibody detection in relation to intracranial haemorrhage (ICH) and the diagnostic difficulty of acute dengue infection
DENV is a flavivirus that is transmitted by urban-dwelling Aedes aegypti and Aedes albopictus mosquitoes. There are four known serotypes of the DENV that are genetically and antigenically linked : DENV 1, 2, 3, and 4 [ 15, 44]. Even though our understanding of dengue infection is improving, no cure has yet been developed, and prevention and control of its mosquito vectors is the most effective strategy. The DENV commonly causes a flu-like illness in children and adults, although much more severe disease symptoms, such as plasma leakage, are possible. Symptoms of an infection include a fever of approximately 104 degrees Fahrenheit, pain behind the eyes, severe headache, muscle/joint pain, vomiting, swollen glands, and rash. These symptoms can last up to 7 days, but typically appear between 4 and 10 days following a mosquito bite. Typically, thrombocytopenia and leukopenia are the first indicators of DF suspicion [ 44, 48]. In dengue cases with more severe symptoms, AST and ALT levels were elevated [ 46]. In dengue-endemic areas, a positive tourniquet test aids in the early diagnosis of dengue infection with a positive predictive value of 70% to 80% [ 57]. However, the sensitivity of the tourniquet test was insufficient for diagnosing acute dengue infection [ 29]. Effective patient management requires a prompt diagnosis. Patients are clinically diagnosed with DENV infection using a variety of ways, such as viral isolation, serologic, and molecular detection. Isolation of the virus is the most reliable indicator of infection. Some laboratories continue to undertake traditional DENV isolation, although it is time-consuming and delays the diagnosis [ 36]. It has been gradually substituted by molecular methods such as nucleic acid amplification tests (NAATs). In comparison to culture-based testing and molecular detection, serological assays are more commonly used to identify dengue infection because to their low cost and ease of use. Other diagnostic tests for DENV detection include viral antigen detection by NS1 and immunological response based tests (IgM and IgG) antibody assays [ 15]. During the acute phase of disease, NAATs provide a diagnosis of DENV on the same or following day. NAATs quantify viral RNA/DNA with greater precision and sensitivity, enabling identification of the acute phase. Within 5 days of the onset of symptoms, the viral genome can be detected. The ability of NAATs to identify viral RNA at the onset of disease is a key advantage. NAATs are also sensitive, specific, rapid, less difficult, and cheaper than virus isolation techniques [ 7]. Although NAATs are quick and precise, they require a laboratory with specific equipment and skilled personnel to do the analysis. In resource-poor, isolated areas where dengue is endemic, these are not always viable options. In primary infection, IgM antibodies are typically detected 3 to 5 days after the onset of symptoms, rise rapidly within 2 weeks, and become undetectable after 2 to 3 months, whereas IgG antibodies are low by the end of the first week, gradually increase, and persist for many years. Meanwhile, during secondary infection, IgG antibodies are detectable at high levels, whereas early convalescent IgM levels are still low, and in some cases undetectable [ 15, 57, 58]. According to one study, no IgM antibody was detected in patients with primary dengue infection on days 1-3 of the disease, but 94% were detected after 7 days [ 49]. Other research found that specific IgM was detected in primary DENV infections after day 6 or later, but not in all secondary infections on days 2 and 3 [ 43]. This finding limits the utility of IgM detection in diagnosing dengue infection during the acute phase of the illness, especially if the patient has complications like ICH. Tests based on IgG can be used to identify past or present illnesses. The IgG antibody is created after the IgM antibody, and IgG antibodies can survive for a prolonged duration, possibly a lifetime [ 30]. It is usual to associate a four-fold increase in IgG antibodies to an acute infection during secondary infection [ 1]. IgG testing can be helpful in some situations, although IgM and NS1 enzyme-linked immunosorbent assay (ELISA) are more beneficial for acute infections. Furthermore, since there is cross-reactivity with other flavivirus IgG antibodies, it is difficult to identify the original infection using IgG-based assays alone [ 15]. Typically, an IgM/IgG ratio analysis differentiates between initial and subsequent infections. Both in IgM and IgG ELISA tests, optical density is assessed. Multiple researchers have demonstrated that Dengue virus IgM/IgG ratio for sera obtained during 30 days of symptoms onset accurately distinguishes patients as having a primary or secondary infection. The discriminating ratios range between 1.2 to 2.0, based on the assays and interpretation techniques of the laboratory [ 37]. The IgG avidity test, especially with modification criteria, has a good ability to distinguish the primary and secondary dengue infection [ 33]. However, Jensenius et al. [ 14] reported a low sensitivity of rapid diagnostic tests that produced negative results in patients with DF. Thus, it is necessary to consider other optimal diagnostic tools. NS1 is a 50 kDa glycoprotein that arises in a variety of oligomeric forms. In the dimeric state, it plays several roles in viral propagation. It is also released by infected cells as a hexamer with such a lipid-filled central channel. Secreted NS1 lipoprotein engages immediately with endothelial or toll-like receptor 4 on myeloid cells to induce the complement system, which may have direct effects on vasculopathy and plasma leakage [ 41]. Because the antigen is generated and released into the bloodstream of infected patients, it is regarded as a crucial biomarker for diagnosing flavivirus infection at an early stage. NS1 assays are essential in clinical settings because they can detect the acute phase of DENV, and because NS1 stays longer than viremia in the blood [ 50]. Typically, lateral flow-based rapid tests are used for rapid detection, whereas antigen-capture ELISAs are utilized for laboratory-based analysis [ 52]. Rapid testing can be performed in situations with limited resources and limited access to adequate health care facilities. All fast tests typically require less than 30 minutes to complete [ 32]. Santoso et al. [ 47] reported that the sensitivity DENV NS1 rapid diagnostic tests for the diagnosis of acute dengue sickness is relatively high, ranging from 73% to 80%, with a specificity of 100%, depending on host factors including age and immunologic state and virus characteristics such as DENV type. Even though the performance of NS1 was shown to be good, numerous groups from various countries found a lower sensitivity of NS1 tests in comparison to molecular approaches, particularly in communities that had multiple outbreaks of this disease in succession [ 9, 12, 47]. As this observation is more evident in secondary infections, it has been proposed that NS1 could be bound to IgG inimmunocomplexes [ 19].
Imaging of the brain
Clinical manifestations of dengue infection are highly variable and include DF, which is only characterized by fever symptoms accompanied by two other symptoms such as headache, retroorbital pain, myalgia, arthralgia/bone pain, rash, and mild bleeding manifestations without plasma leakage, dengue haemorrhagic fever with symptoms resembling DF and plasma leakage, and expanded dengue syndrome, which is characterized by unusual dengue symptoms. This uncommon manifestation in DHF patients was associated with prolonged shock with organ failure or patients with co-infections [ 57, 58]. In cases of DF, ICH is uncommon but a significant challenge for clinicians [ 5]. In cases of acute neurologic deficit, head computed tomography (CT) has traditionally been the imaging modality of choice. Head CT has the advantages of being a quick, readily available, and generally less expensive neuroimaging modality. There are currently no guidelines regarding when a head CT should be performed on dengue patients; however, early radiological diagnosis may serve as a good prognostic indicator [ 10, 44]. Patients suspected of having ICH due to DF may exhibit headache, fever, and vomiting. Possible additional symptoms include weakness, numbness, loss of consciousness, and seizures [ 44]. Due to the impossibility of recommending head CT screening for all dengue patients, CT should only be considered when clinical findings indicate a high index of suspicion [ 5]. Especially, patients with secondary dengue infections who have positive IgG antibodies are at a higher risk of ICH and have a poor prognosis, and should be monitored with lower diagnostic CT thresholds for the brain [ 44]. Rapid-sequence magnetic resonance imaging (rsMRI) of the brain has gained popularity as an alternative to head CT in children due to the rapid recording of images [ 31]. The advantages of rsMRI for detecting dysfunction of ventriculoperitoneal shunt and as a screening tool for non-accidental damage have also been studied previously [ 35]. Although these examinations are ŌĆ£quick,ŌĆØ they still require more time than a head CT without contrast [ 6, 59]. Ramgopal et al. [ 40] also discovered that a greater proportion of rsMRI examinations failed compared to head CT, primarily due to patient motion. Cost is also frequently cited as a barrier to the use of MRI as opposed to CT. Lastly, it is crucial to implement MRI sequences that correspond to the intended diagnosis. In a study describing a FIESTA-based procedure, Rozovsky et al. [ 42] discovered a significant false negative rate, including a missed venous sinus thrombosis and subdural bleeding. With these limitations in mind, rsMRI may be a viable alternative to head CT, particularly for pediatric dengue patients with acute neurologic abnormalities.
Possible etiology of ICH
Complex mechanisms underlie the susceptibility of dengue-infected patients to ICH. The pathophysiology of neurological involvement includes virus-induced direct tissue lesions, capillary haemorrhage, disseminated intravascular coagulation, metabolic disorders, fulminant liver failure, and cerebral edema caused by increased vascular permeability [ 54]. Dengue causes intracerebral haemorrhage due to thrombocytopenia and abnormal bleeding parameters [ 21]. The proposed mechanism underlying intracerebral haemorrhage in dengue infection is primarily associated with haemostatic disorders. Infection with the DENV can result in vascular activity, capillary leakage, an increase in fibrinolysis, and the release of mediators in conjunction with bleeding. Due to immunopathological mechanisms, the presence of DF antibodies and antigens in CSF indicates blood-brain barrier and blood-CSF barrier breaches, as well as vasculopathy [ 39]. Significant effects of viral pathogenesis on ICH and infarction have also been observed [ 5].
Risk factors as predictors of ICH in DF
ICH is rarely reported in dengue patients. Therefore, it is highly advantageous to prevent ICH in dengue patients and rapidly identify patients with severe bleeding before surgical and medical interventions are no longer possible [ 44]. The most accurate predictors of bleeding, according to a study, are a prolonged duration of shock and a haematocrit that is lower than normal during shock [ 25]. In contrast, other study concluded that a combination of biphasic fever patterns, haemoconcentration, thrombocytopenia (<50000/mm 3), and elevated ALT had a 70% positive predictive value and a 75% negative predictive value for predicting spontaneous bleeding in dengue [ 54]. Jayasinghe et al. [ 13] reported that a platelet count below 40000/mm 3 was associated with multiple cerebral haemorrhages and subdural hematoma in patients with DF. Talukdar et al. [ 55] identified three risk factors for plasma leakage : body mass index Ōēź25.0 kg/m 2, platelet count <100000/mm 3 on fever days 3 to 4, and elevated AST and ALT (Ōēź100 U/I) on fever days 3 or 4. Previous study also reported that older patients, those with secondary infection, elevated haematocrit levels, low platelet counts, prolonged activated partial thromboplastin time, and female gender were at an increased risk for bleeding; however, Chang et al.5) discovered that altered consciousness and a higher Charlson comorbidity score (>4) are significant risk factors for intracranial bleeding in dengue [ 4]. According to a study, three patients with positive IgG who required emergency surgery died, whereas two patients with negative IgG did not have ICH requiring surgery and survived [ 44]. Consequently, secondary infections may be linked to severe ICH.
Neurosurgical procedure consideration
Whether or not dengue patients with ICH should undergo neurosurgical intervention is still up for debate. Surgical treatment of dengue infection with ICH is frequently delayed and difficult because vascular disorders, coagulation disorders, and platelet dysfunction necessitate blood transfusions to correct platelets and other coagulation parameters [ 44]. Other factors, such as the availability of neurosurgical centres, the experience of the surgeon, the severity of the disease, the presence of comorbid conditions, and the distance for transporting critically ill patients, can influence the outcome [ 53]. Kumar et al.22) reported that prompt and timely surgical intervention resulted in a good recovery despite a poor neurologic status prior to surgery. Gautam et al. [ 10] also reported that early neurosurgical interventions performed under balanced anesthesia on patients in need are a good prognostic indicator. Several studies have also reported the success of surgical intervention in the treatment of ICH in dengue [ 10, 23, 26, 51, 54, 56]. However, Sam et al. [ 44] reported that two surgical patients died from excessive bleeding during the procedure. Typically, patients who received a conservative intervention due to low platelet counts. Due to the increased risk of hemorrhagic complications following surgery, the recommended platelet count prior to neurosurgical intervention is 100000/mm 3 [ 24]. Nevertheless, Kutty et al. [ 23] reported an emergency neurosurgical intervention when the platelet count was 40000/mm 3 and multiple blood transfusions of fresh blood, plasma, and platelets were administered during surgery. Due to the fact that the outcome of neurosurgical procedures depends on numerous variables, such as platelet counts, coagulation profile, haematocrit, location of intracranial bleeds, associated complications, and the presence of shock, as well as the facilities and neurosurgeonŌĆÖs experiences, additional research is required before neurosurgical interventions can be considered. Additionally, improved outcomes were associated with surgical interventions performed within 8 hours of the onset of bleeding [ 11, 28]. Thus, it may appear that prompt surgical intervention is advantageous in the management of ICH, but it is necessary to confirm patientsŌĆÖ conditions prior to performing emergency surgery in the management of ICH in DF.
Recommendation for transfusion of platelets and fresh frozen plasma
Patients with dengue-associated ICH often suffer from severe thrombocytopenia and coagulopathy, which makes it difficult for neurosurgeons and physicians to set appropriate target levels and transfuse the appropriate amount of blood products before surgery [ 44]. Controversy surrounds the effectiveness of platelet and fresh-frozen plasma transfusions in preventing ICH and their role in ICH patients with dengue [ 53]. Gautam et al. [ 10] discovered, however, that early effective intravenous fluid supportive therapy, anti-edema agents, and platelet transfusion may be an indicator of a favorable prognosis in the management of ICH in DF. For neurosurgical procedures, the recommended platelet count is 100├Ś10 9/L [ 8]. It is challenging to maintain the recommended perioperative platelet levels in dengue patients, and it has been shown that a perioperative platelet count below 100000/┬ĄL in patients who did not respond to platelet transfusions increased the risk of postoperative hemorrhagic complications [ 24]. In order to prevent hemorrhagic complications, DF with thrombocytopenia is treated with prophylactic platelet transfusions, as opposed to therapeutic platelet transfusions, which are administered to patients with clinical bleeding [ 17]. The AABB also recommends transfusing hospitalized adult patients with a platelet count of 10├Ś10 9/L or less with a single apheresis unit or equivalent to reduce the risk of spontaneous bleeding [ 16]. A recent randomized controlled trial in Singapore and Malaysia found a significant interaction between platelet recovery and transfusion in adult dengue with thrombocytopenia. Patients with negative platelet recuperation have been much more likely to bleed if given a prophylactic platelet transfusions [ 2]. The efficacy of prophylactic platelet transfusion in dengue, as well as the precise trigger for platelet transfusions, are also contested. In the absence of bleeding symptoms, the guidelines for the management of dengue in India recommend a trigger of 10000/mm 3 and state that prophylactic platelet transfusions are not necessary for stable patients with platelet counts below 20000/mm 3 [ 17]. It has been reported that highrisk patients with a platelet count below 20000/mm 3 who are at risk of bleeding require an urgent platelet transfusion [ 27]. The prophylactic administration of platelets and fresh frozen plasma transfusions has proven ineffective. Instead, these transfusions result in fluid overload and prolonged hospitalization [ 25]. Due to the involvement of complex multifactorial pathogenic mechanisms in dengue haemorrhagic symptoms, monitoring of platelet counts and routine coagulation profiles are insufficient, and a global evaluation of the efficacy of haemostatic mechanisms is required [ 53]. Other methods, including thromboelastometry and platelet aggregometry, may also be used to determine the need for prophylactic blood transfusions. These studies have validated targeted transfusion therapies in patients with bleeding after major surgery and trauma, as well as in patients with hemorrhagic haemophilia; however, their role in patients with ICH due to DF remains unclear [ 44].
CONCLUSION
ICH is an uncommon DF complication. Thrombocytopenia and secondary infections may both be risk factors for ICH. When ICH symptoms are suspected, a prompt diagnosis requires laboratory results and brain imaging. Considering emergency surgery while monitoring thrombocytopenia may be a treatment for ICH in DF. However, before neurosurgical intervention recommendations for thrombocytopenia patients can be made, larger-scale research could be conducted.
Fig.┬Ā1.
PRISMA of concluded study. PRISMA : Preferred Reporting Items for Systematic Reviews and Meta-Analyses. 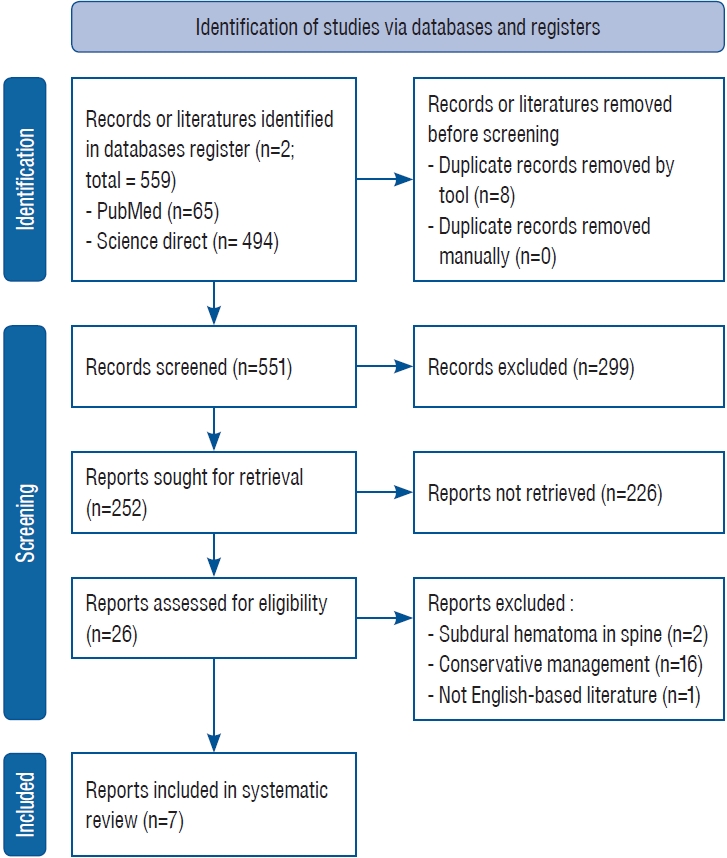
Table┬Ā1.
Characteristics of the included study
|
Study |
Patient |
Laboratory finding
|
Types of ICH |
Detection of ICH after fever onset |
Intervention |
Outcome |
|
Thrombocyte on ICH onset*
|
AST/ALT on ICH onsetŌĆĀ
|
Antigen/antibody finding |
|
Ashraf et al. [3] (2022) |
A 65-year-old male |
26.000 |
111/51 |
NS1 antigen, IgM and IgG positive |
SDH |
8 |
Surgical |
Recovered |
|
Siahaan et al. [51] (2022) |
A 65-year-old male |
42.000 |
242/252 |
NS1 antigen and IgG positive |
Right SDH |
5 |
Surgical |
Recovered |
|
Kutty et al. [23] (2019) |
A 24-year-old female |
19.000 |
Not mentioned |
NS1 antigen and IgM positive |
Left frontal EDH |
10 |
Surgical |
Recovered |
|
Sam et al. [45] (2018) |
A 47-year-old male |
31.000 |
Not mentioned |
NS1 antigen and IgG positive |
Right SDH |
7 |
Surgical |
Death |
|
Gautam et al. [10] (2016) |
A 37-year-old male |
40.700 |
Not mentioned |
Positive serology (not mentioned) |
EDH |
Not mentioned |
Surgical |
Recovered |
|
Gautam et al. [10] (2016) |
A 50-year-old male |
55.000 |
Not mentioned |
Positive serology (not mentioned) |
EDH |
Not mentioned |
Surgical |
Recovered |
|
Gautam et al. [10] (2016) |
A 25-year-old male |
25.700 |
Not mentioned |
Positive serology (not mentioned) |
Acute SDH |
Not mentioned |
Surgical |
Recovered |
|
Gautam et al. [10] (2016) |
A 50-year-old male |
35.000 |
Not mentioned |
Positive serology (not mentioned) |
Chronic SDH |
Not mentioned |
Surgical |
Recovered |
|
Gautam et al. [10] (2016) |
A 14-year-old male |
18.000 |
Not mentioned |
Positive serology (not mentioned) |
ICH |
Not mentioned |
Surgical |
Death |
|
Gautam et al. [10] (2016) |
A 44-year-old male |
25.200 |
Not mentioned |
Positive serology (not mentioned) |
Chronic SDH |
Not mentioned |
Surgical |
Recovered |
|
Sam et al. [44] (2016) |
A 47-year-old male |
31.000 |
-/65 |
NS1 antigen and IgG positive |
Right convexity SDH |
7 |
Surgical |
Death |
|
Sam et al. [44] (2016) |
A 54-year-old male |
24.000 |
-/44 |
NS1 and IgM positive |
Right convexity SDH |
7 |
Surgical |
Death |
|
Kumar et al. [22] (2009) |
A 9-year-old male |
26.000 |
Not mentioned |
Positive serology (not mentioned) |
Left frontoparietal SDH |
7 |
Surgical |
Recovered |
|
Kumar et al. [22] (2009) |
A 40-year-old male |
70.000 |
Not mentioned |
Positive serology (not mentioned) |
Right frontoparietal SDH |
5 |
Surgical |
Recovered |
References
1. Andries AC, Duong V, Ngan C, Ong S, Huy R, Sroin KK, et al : Field evaluation and impact on clinical management of a rapid diagnostic kit that detects dengue NS1, IgM and IgG. PLoS Negl Trop Dis 6 : e1993, 2012    2. Archuleta S, Chia PY, Wei Y, Syed-Omar SF, Low JG, Oh HM, et al : Predictors and clinical outcomes of poor platelet recovery in adult dengue with thrombocytopenia: a multicenter, prospective study. Clin Infect Dis 71 : 383-389, 2020    3. Ashraf M, Hussain SS, Farooq M, Fatima L, Majeed N, Ashraf N : Isolated subdural hematoma due to dengue hemorrhagic fever: surgical intervention and review of the literature. Surg Neurol Int 13 : 244, 2022    4. Chamnanchanunt S, Kanagaraj D, Thanachartwet V, Desakorn V, Rojnuckarin P : Early predictors of clinically significant bleeding in adults with dengue infection. Southeast Asian J Trop Med Public Health 43 : 890-899, 2012  5. Chang K, Huang CH, Chen TC, Lin CY, Lu PL, Chen YH : Clinical characteristics and risk factors for intracranial hemorrhage or infarction in patients with dengue. J Microbiol Immunol Infect 54 : 885-892, 2021   6. Cohen AR, Caruso P, Duhaime AC, Klig JE : Feasibility of ŌĆ£rapidŌĆØ magnetic resonance imaging in pediatric acute head injury. Am J Emerg Med 33 : 887-890, 2015   7. Deubel V, Laille M, Hugnot JP, Chungue E, Guesdon JL, Drouet MT, et al : Identification of dengue sequences by genomic amplification: rapid diagnosis of dengue virus serotypes in peripheral blood. J Virol Methods 30 : 41-54, 1990   8. Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al : Guidelines for the use of platelet transfusions. Br J Haematol 176 : 365-394, 2017    10. Gautam S, Meena RK, Meena SC, Gautam B : Retrospective analysis of prognostic factors in dengue infected patients with intracranial bleed. Surg Neurol Int 7( Suppl 39):S935-S939, 2016    11. Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S, et al : Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke 43 : 1496-1504, 2012    12. Guzman MG, Jaenisch T, Gaczkowski R, Ty Hang VT, Sekaran SD, Kroeger A, et al : Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis 4 : e811, 2010    13. Jayasinghe NS, Thalagala E, Wattegama M, Thirumavalavan K : Dengue fever with diffuse cerebral hemorrhages, subdural hematoma and cranial diabetes insipidus. BMC Res Notes 9 : 265, 2016    14. Jensenius M, Berild D, Ormaasen V, Maehlen J, Lindegren G, Falk KI : Fatal subarachnoidal haemorrhage in a Norwegian traveller with dengue virus infection. Scand J Infect Dis 39 : 272-274, 2007   15. Kabir MA, Zilouchian H, Younas MA, Asghar W : Dengue detection: advances in diagnostic tools from conventional technology to point of care. Biosensors (Basel) 11 : 206, 2021    16. Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al : Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 162 : 205-213, 2015   17. Kaur P, Kaur G : Transfusion support in patients with dengue fever. Int J Appl Basic Med Res 4( Suppl 1):S8-S12, 2014    20. Kularatne SA, Dalugama C : Dengue infection: global importance, immunopathology and management. Clin Med (Lond) 22 : 9-13, 2022    21. Kulkarni R, Pujari S, Gupta D : Neurological manifestations of dengue fever. Ann Indian Acad Neurol 24 : 693-702, 2021    22. Kumar R, Prakash O, Sharma BS : Intracranial hemorrhage in dengue fever: management and outcome: a series of 5 cases and review of literature. Surg Neurol 72 : 429-433; discussion 433, 2009  23. Kutty RK, Sreemathyamma SB, Sivanandapanicker JL, Mundhe V, Chhabra K, Peethambaran A : Burden of dengue-related neurosurgical emergencies during an epidemic: a tertiary care experience. Asian J Neurosurg 14 : 211-218, 2019    24. Li D, Glor T, Jones GA : Thrombocytopenia and neurosurgery: a literature review. World Neurosurg 106 : 277-280, 2017   25. Lum LC, Abdel-Latif Mel-A, Goh AY, Chan PW, Lam SK : Preventive transfusion in Dengue shock syndrome-is it necessary? J Pediatr 143 : 682-684, 2003   26. Mahale RR, Mehta A, Shankar AK, Srinivasa R : Delayed subdural hematoma after recovery from dengue shock syndrome. J Neurosci Rural Pract 7 : 323-324, 2016    27. Makroo RN, Raina V, Kumar P, Kanth RK : Role of platelet transfusion in the management of dengue patients in a tertiary care hospital. Asian J Transfus Sci 1 : 4-7, 2007    28. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al : Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 382 : 397-408, 2013    29. Muller DA, Depelsenaire AC, Young PR : Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis 215( suppl_2):S89-S95, 2017   30. Narayan R, Raja S, Kumar S, Sambasivam M, Jagadeesan R, Arunagiri K, et al : A novel indirect ELISA for diagnosis of dengue fever. Indian J Med Res 144 : 128-133, 2016    31. Niederhauser BD, McDonald RJ, Eckel LJ, Keating GF, Broomall EM, Wetjen NM, et al : Retrospective review of rapid pediatric brain MR imaging at an academic institution including practice trends and factors affecting scan times. AJNR Am J Neuroradiol 34 : 1836-1840, 2013    32. Pal S, Dauner AL, Mitra I, Forshey BM, Garcia P, Morrison AC, et al : Evaluation of dengue NS1 antigen rapid tests and ELISA kits using clinical samples. PLoS One 9 : e113411, 2014    33. Palabodeewat S, Masrinoul P, Yoksan S, Auewarakul P, Komaikul J : A modified IgG avidity assay for reliability improvement of an in-house capture ELISA to discriminate primary from secondary dengue virus infections. J Virol Methods 289 : 114043, 2021   34. Patel AB, Ranade D, More B, Lachke A : A rare case of spontaneous subdural hemorrhage in dengue fever that mimics a tumor on MRI: a case report. Egypt Spine J 39 : 76-80, 2021  35. Patel DM, Tubbs RS, Pate G, Johnston JM Jr, Blount JP : Fast-sequence MRI studies for surveillance imaging in pediatric hydrocephalus. J Neurosurg Pediatr 13 : 440-447, 2014   36. Pervin M, Tabassum S, Kumar Sil B, Islam MN : Isolation and serotyping of dengue viruses by mosquito inoculation and cell culture technique: an experience in Bangladesh. Dengue Bulletin 27 : 81-90, 2003
38. Prompetchara E, Ketloy C, Thomas SJ, Ruxrungtham K : Dengue vaccine: global development update. Asian Pac J Allergy Immunol 38 : 178-185, 2020  39. Puccioni-Sohler M, Rosadas C, Cabral-Castro MJ : Neurological complications in dengue infection: a review for clinical practice. Arq Neuropsiquiatr 71 : 667-671, 2013   41. Rastogi M, Sharma N, Singh SK : Flavivirus NS1: a multifaceted enigmatic viral protein. Virol J 13 : 131, 2016    42. Rozovsky K, Ventureyra EC, Miller E : Fast-brain MRI in children is quick, without sedation, and radiation-free, but beware of limitations. J Clin Neurosci 20 : 400-405, 2013   43. Sa-Ngasang A, Anantapreecha S, A-Nuegoonpipat A, Chanama S, Wibulwattanakij S, Pattanakul K, et al : Specific IgM and IgG responses in primary and secondary dengue virus infections determined by enzymelinked immunosorbent assay. Epidemiol Infect 134 : 820-825, 2006    44. Sam JE, Gee TS, Nasser AW : Deadly intracranial bleed in patients with dengue fever: a series of nine patients and review of literature. J Neurosci Rural Pract 7 : 423-434, 2016   45. Sam JE, Gee TS, Wahab NA : Fatal intracranial hemorrhage in a patient with severe dengue fever. Asian J Neurosurg 13 : 56-58, 2018    46. Samanta J, Sharma V : Dengue and its effects on liver. World J Clin Cases 3 : 125-131, 2015    47. Santoso MS, Yohan B, Denis D, Hayati RF, Haryanto S, Trianty L, et al : Diagnostic accuracy of 5 different brands of dengue virus non-structural protein 1 (NS1) antigen rapid diagnostic tests (RDT) in Indonesia. Diagn Microbiol Infect Dis 98 : 115116, 2020   48. Schaefer TJ, Panda PK, Wolford RW : Dengue Fever : StatPearls. Treasure Island : StatPearls Publishing, 2022
49. Schilling S, Ludolfs D, Van An L, Schmitz H : Laboratory diagnosis of primary and secondary dengue infection. J Clin Virol 31 : 179-184, 2004   50. Shenoy B, Menon A, Biradar S : Diagnostic utility of dengue NS1 antigen. Pediatr Infect Dis 6 : 110-113, 2014  51. Siahaan AMP, Tandean S, Saragih EB, Nainggolan BWM : Spontaneous acute subdural hematoma in dengue fever: case report and review of the literature. Int J Surg Case Rep 98 : 107512, 2022    52. Sinawang PD, Rai V, Ionescu RE, Marks RS : Electrochemical lateral flow immunosensor for detection and quantification of dengue NS1 protein. Biosens Bioelectron 77 : 400-408, 2016   53. Singh A, Balasubramanian V, Gupta N : Spontaneous intracranial hemorrhage associated with dengue fever: an emerging concern for general physicians. J Family Med Prim Care 7 : 618-628, 2018    54. Sunder S, Prasad R : Acute subdural hematoma in a patient with dengue - case report and review of literature. Apollo Med 5 : 125-129, 2008  55. Talukdar S, Thanachartwet V, Desakorn V, Chamnanchanunt S, Sahassananda D, Vangveeravong M, et al : Predictors of plasma leakage among dengue patients in Thailand: a plasma-leak score analysis. PLoS One 16 : e0255358, 2021    56. Wafa SR, Jamsari S, Karis BM : A case report: intracranial haemorrhage in a patient with probable dengue fever. Med J Malaysia 54 : 273-276, 1999  57. World Health Organization (WHO) : Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. Geneva : WHO, 2011
58. World Health Organization (WHO) : Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva : WHO, 2009
59. Yue EL, Meckler GD, Fleischman RJ, Selden NR, Bardo DM, Chu OŌĆÖConnor AK, et al : Test characteristics of quick brain MRI for shunt evaluation in children: an alternative modality to avoid radiation. J Neurosurg Pediatr 15 : 420-426, 2015  
|
|

















