Lee, Jo, Kim, and Park: Metastatic Brain Neuroendocrine Tumor Originating from the Liver
Abstract
A 67-year-old male presented with left temporal hemianopsia and left hemiparesis. A contrast-enhanced magnetic resonance image revealed a 4.5├Ś3.5├Ś5.0 cm rim-enhancing mass with central necrosis and associated edema located in the left occipital lobe. Of positron emission tomography and abdominal computed tomography, a 9-cm mass with poor enhancement was found in the right hepatic lobe. Craniotomy and right hemihepatectomy was performed. The resected specimen showed histological features and immunochemical staining consistent with a metastatic neuroendocrine tumor (NET). Four months later, the tumors recurred in the brain, liverand spinal cord. Palliative chemotherapy with etoposide and cisplatin led to complete remission of recurred lesions, but the patient died for pneumonia. This is the first case of a metastatic brain NET originating from the liver. If the metastatic NET of brain is suspicious, investigation for primary lesion should be considered including liver.
Key Words: Liver ┬Ę Neuroendocrine tumor ┬Ę Treatment ┬Ę Chemotherapy.
INTRODUCTION
In patients with neuroendocrine tumors (NETs), the incidence of brain metastasis is rare, with estimates between 1.5-5% 4). NETs of the liver are extremely rare. To our knowledge, this is the first case of primary hepatic NET with brain metastasis. Clinical diagnosis of metastatic brain NETs is usually difficult because of the rarity of the tumor and the difficulty of differentiating it from other brain tumors like glioblastoma multiforme through imaging study. Since the prognosis of glioblastoma multiforme is poor due to its high-grade status, prompt surgery for pathologic confirmation and resection is warranted 2,16). And if the NET of brain is confirmed, investigation for primary lesion should be considered including liver.
CASE REPORT
History and examination
A 67-year-old male presented with headache, left temporal hemianopsia and progressive left hemiparesis beginning 10 days prior. Physical examination revealed decreased upper arm motor power to grade IV and lower leg power to grade II. A T1-weighted image from a contrast-enhanced magnetic resonance imaging (MRI) of the brain showed a 4.5├Ś3.5├Ś5.0 cm rim-enhancing mass with central necrosis and associated edema located in the right occipital lobe ( Fig. 1A).
Operation
The patient underwent a craniotomy for diagnosis and symptom relief. Histological features and immunohistochemistry of the tumor were consistent with a metastatic NET. The tumor cells were positive for CD56, synaptophysin ( Fig. 2) and negative for glial fibrillary acidic protein (GFAP). There was necrosis with 50% of Ki-67 labeling index, so it was considered a poorly differentiated neuroendocrine carcinoma (G3 by 2010 WHO classification). Positron emission tomography-computed tomography (PET-CT) and liver MRI confirmed the presence of a 9├Ś10├Ś8.5 cm mass in the right hepatic lobe ( Fig. 1B, C). The specimen of the sono-guided liver biopsy demonstrated the same histological findings as the brain lesion. We could not find another primary focus, so we concluded that the patient had primary hepatic neuroendocrine carcinoma with brain metastasis. Right hemihepatectomy with cholecystectomy was then performed. The tumor size was 10├Ś10 cm with neuroendocrine tumor, and the resection margins were negative.
Adjuvant treatment
Then the patient was followed on a regular basis in outpatient clinic. About 4 months after craniotomy, the patient complained of progressive left-sided weakness. Restaging was performed. Brain-enhanced MRI revealed a suspicious recurrence in the right occipital lobe and right inferior aspect of the cerebellum ( Fig. 3A). Enhanced CT of the abdomen revealed a suspicious recurrence in segment 8 of the liver ( Fig. 3B). We resected the recurrent tumor mass in the right occipital lobe to relieve the progressive neurologic symptoms. The patient then received postoperative cranial radiation. After radiation therapy, he complained of bilateral leg weakness. PET-CT scan and spine-enhanced MRI showed multifocal FDG uptake in the thoracic and lumbar spinal cord ( Fig. 4). We decided to recommend palliative chemotherapy with etoposide and cisplatin. The chemotherapy was delivered every 28 days with the following regimen : on day 1, etoposide 100 mg/m 2 over 1 hour I.V. infusion; on days 2 and 3, etoposide 100 mg/m 2 over 1 hour I.V. infusion and cisplatin 45 mg/m 2 over 24 hour I.V. infusion. About two months after the third cycle of etoposide and cisplatin chemotherapy, PET-CT, brain-enhanced MRI, spine-enhanced MRI and abdomen-enhanced CT showed complete remission of the previously enhancing tumor ( Fig. 5). Unfortunately, paralysis of the lower extremities was not improved. About 3 weeks after discharge, the patient was readmitted for fever and purulent sputum. Despite intensive treatment including ventilator care, the patient died after 1 month from pneumonia.
DISCUSSION
Among NETs, primary hepatic NETs are extremely rare 3,56). As the liver is the most frequent metastatic site for NETs, the differential diagnosis between NET and metastatic hepatic neuroendocrine carcinoma is often difficult and debatable 1,1114). In previous studies, NETs occur most often in middle-aged and female patients. The most common presenting symptom is abdominal pain, and about 5% of patients present with typical carcinoid syndrome 6,10). Usually, the prognosis of a poorly differentiated NET is very poor, with a median survival of approximately 6 months without therapy 8,1315). In 1991, Moertel et al. 12) reported an overall response rate of 67% (17% complete remission, 50% partial remission), with a median progression-free survival of 8 months and a median overall survival of 19 months in 18 patients diagnosed with undifferentiated NETs. Until now, the treatment of choice for localized, primary hepatic NET is hepatectomy 9,17). Surgical resection of a primary liver tumor is necessary for symptom relief by cytoreduction and possible cure. As in this case, one could consider metastectomy of the brain for both diagnosis and treatment if the brain lesion is resectable. For patients with NETs and multiple masses or distant metastases, chemotherapy is an option. According to the one study of primary NETs arising from the hepatobiliary and pancreatic regions, a response rate of 14% and median survival of 5.8 months were obtained in response to combined etoposide plus cisplatin therapy 7).
CONCLUSION
In conclusion, if the brain imaging of the patient has the possibility of metastasis, systemic evaluation is warranted. This case is first report for the primary liver NET with brain metastasis. We should remember that brain NET should be confirmed by pathology, and rarely liver NET could be primary.
References
1. Akahoshi T, Higashi H, Tsuruta S, Tahara K, Matsumoto T, Takeuchi H, et al : Primary neuroendocrine carcinoma coexisting with hemangioma in the liver : report of a case. Surg Today 2010, 40 : 185-189,   2. Burger PC, Green SB : Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer 1987, 59 : 1617-1625,   3. Donadon M, Torzilli G, Palmisano A, Del Fabbro D, Panizzo V, Maggioni M, et al : Liver resection for primary hepatic neuroendocrine tumours : report of three cases and review of the literature. Eur J Surg Oncol 2006, 32 : 325-328,   4. Hlatky R, Suki D, Sawaya R : Carcinoid metastasis to the brain. Cancer 2004, 101 : 2605-2613,   5. Huang YQ, Xu F, Yang JM, Huang B : Primary hepatic neuroendocrine carcinoma : clinical analysis of 11 cases. Hepatobiliary Pancreat Dis Int 2010, 9 : 44-48,  6. Iwao M, Nakamuta M, Enjoji M, Kubo H, Fukutomi T, Tanabe Y, et al : Primary hepatic carcinoid tumor : case report and review of 53 cases. Med Sci Monit 2001, 7 : 746-750,  7. Iwasa S, Morizane C, Okusaka T, Ueno H, Ikeda M, Kondo S, et al : Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol 2010, 40 : 313-318,   8. Johnson LA, Lavin P, Moertel CG, Weiland L, Dayal Y, Doos WG, et al : Carcinoids : the association of histologic growth pattern and survival. Cancer 1983, 51 : 882-889,   9. Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW : Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol 2003, 10 : 1171-1175,   10. Lin CW, Lai CH, Hsu CC, Hsu CT, Hsieh PM, Hung KC, et al : Primary hepatic carcinoid tumor : a case report and review of the literature. Cases J 2009, 2 : 90,    11. Modlin IM, Lye KD, Kidd M : A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97 : 934-959,   12. Moertel CG, Kvols LK, O'Connell MJ, Rubin J : Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991, 68 : 227-232,   13. Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E : Gastric carcinoids and neuroendocrine carcinomas : pathogenesis, pathology, and behavior. World J Surg 1996, 20 : 168-172,   14. R├╝ckert RI, R├╝ckert JC, D├Črffel Y, Rudolph B, M├╝ller JM : Primary hepatic neuroendocrine tumor : successful hepatectomy in two cases and review of the literature. Digestion 1999, 60 : 110-116,   15. Staren ED, Gould VE, Warren WH, Wool NL, Bines S, Baker J, et al : Neuroendocrine carcinomas of the colon and rectum : a clinicopathologic evaluation. Surgery 1988, 104 : 1080-1089,  16. Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC : Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004 : analysis of 625 cases. Br J Neurosurg 2007, 21 : 496-500,   17. Zhang A, Xiang J, Zhang M, Zheng S : Primary hepatic carcinoid tumours : clinical features with an emphasis on carcinoid syndrome and recurrence. J Int Med Res 2008, 36 : 848-859,  
Fig.┬Ā1
A : Axial, T1-weighted, post-gadolinium image of the brain. B : Positron emission tomography-computed tomography (PET-CT). C : Axial, T1-weighted, post-gadolinium image of the liver. Note a rim-enhancing mass with central necrosis and associated edema in the right occipital lobe of brain and right hepatic lobe.

Fig.┬Ā2
Histopathologic findings of brain and liver mass show a round to oval stippled and scant, pink dranular cytoplasm with H&E (A and B) and brain mass are strongly stained with CD56 (C). This finding reveals that brain mass is a neuroendocrine tumor which is the same pathology with liver tissue.

Fig.┬Ā3
A : Axial, T1-weighted, post-gadolinium image of the brain. B : Abdomen enhanced CT at 4 months after first craniotomy and hepatectomy. Note suspicious recurrence in the occipital lobe, inferior right cerebellum, and segment 8 of the liver.
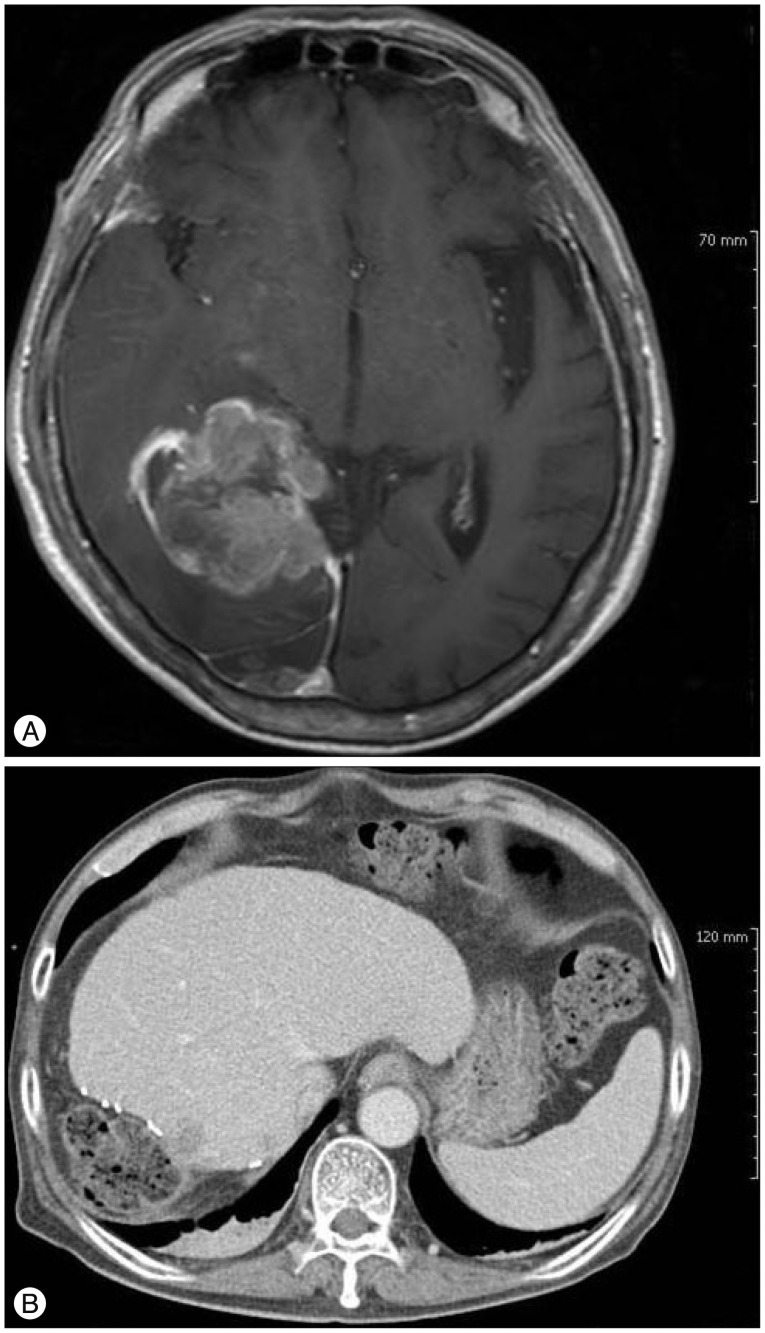
Fig.┬Ā4
A : PET-CT scan after whole-brain radiation therapy. Note the multifocal FDG uptake in the cervical, thoracic and lumbar spinal cord and malignant tumor in right hepatectomy state. B : Spine post-gadolinium image. Note metastasis along the meninges at the T12-L1 level and cauda equine along the lumbar spine.
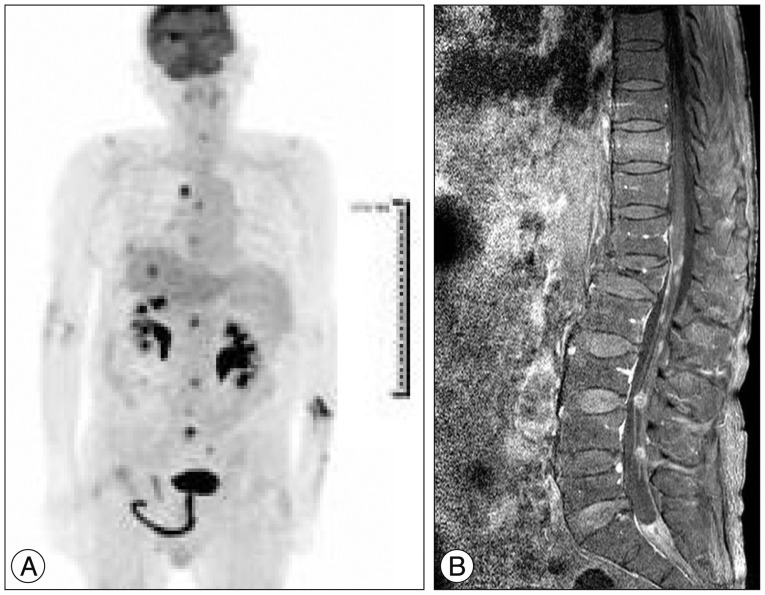
Fig.┬Ā5
A : PET-CT. B : spine post-gadolinium image. C : T1-weighted, post-gadolinium image of the brain. D : Abdomen enhanced CT. Note that previously enhancing tumors have disappeared.

|
|




















