INTRODUCTION
Ankylosing spondylitis (AS) is a seronegative spondyloarthropathy primarily affecting the spine and sacroiliac joint. Neurologic sequele of AS is usually accompanied by an atlantoaxial subluxation with spinal cord compression or a pathologic fracture of the rigid spine at cervicothoracic or thoracolumbar junctions even after a minor traumatic event21). The cauda equine syndrome (CES), is a known, but rare neurologic manifestation in patients with long-standing AS (also called CES-AS syndrome) and was first described by Bowie and Glasgow5) and Hauge9) in 1961.
A dural ectasia is a condition in which the spinal dural sac is enlarged usually involving the lumbosacral regions where the cerebrospinal fluid (CSF) pressure is greatest19). The association of CES with dural ectasia or arachnoid cysts in AS was first recognized by Matthews in 196813). Dural ectasia is occasionally seen in patients with Marfan syndrome, neurofibromatosis Type I, Ehlers-Danlos syndrome and longstanding AS12). However, the bony erosion in AS predominantly involves the posterior elements rather than the posterior aspect of vertebral bodies seen in other conditions14).
CASE REPORT
A 68-year-old male with a 30-year history of AS presented with a 3-month history of aggravation of a progressive urinary incontinence and pain in the left buttock associated with paresthesia of the right buttock which radiated down the back of the leg to the level of the sole. He also presented a three-year history of insidious onset, slowly progressing, urinary incontinence and numbness along the heel and sole of the left foot. On physical examination, the left foot showed a grade IV weakness with an atrophy of the sole. Mild hypesthesia and paresthesia was found in his left posterior calf and sole along the S1 dermatome. The left ankle jerk was diminished. No mechanical allodynia was noticed. The patient had experienced intermittent low back pain in the past, but was not currently asymptomatic regarding this for many years.
On admission, laboratory values were as follows : erythrocyte sedimentation rate 22 (0-15) mm/hr/C-reactive protein 0.23 (range 0.03-0.47) mg/L and negative Anti-Nuclear Ab 2.5. Electromyography and nerve conduction study showed bilateral lumbosacral radiculopathies, clinically equivalent to a cauda equina syndrome, with a more severe involvement of the left side. An urodynamic study showed an evidence of a neurogenic bladder with detrusor hypoactivity and low compliance of the bladder.
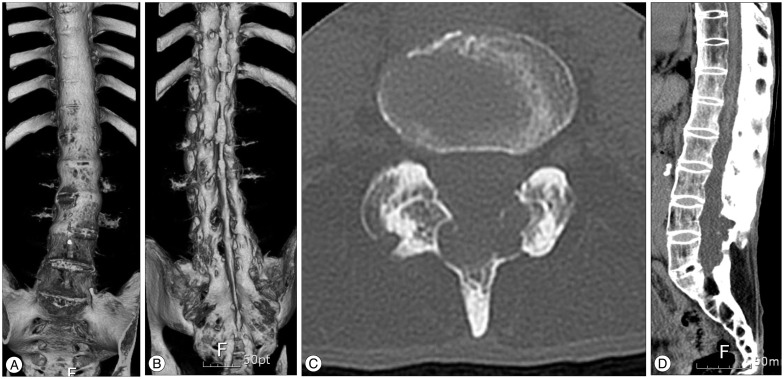
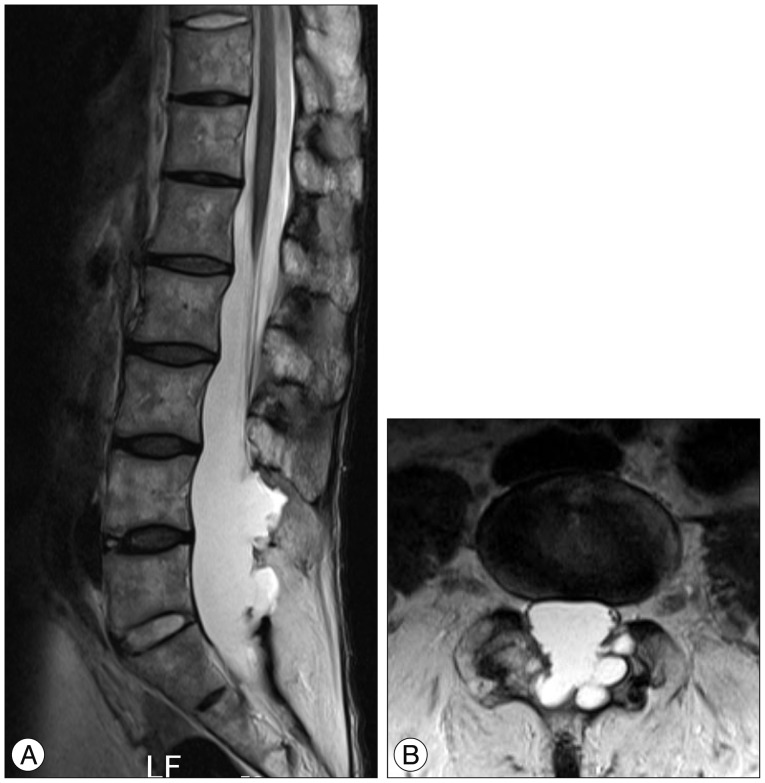
Plain X-rays of the lumbosacral spine and subsequent 3D computed tomography (CT) showed the typical 'bamboo spine' of AS with fusion of both sacroiliac joints, squaring of the lumbar vertebral bodies, syndesmophyte formation along the lumbar spine, and also multiple erosions of the roof of an enlarged spinal canal (Fig. 1A, B, C). A CT scan showed the spinal canal enlarged by laminar erosions (Fig. 1D). The magnetic resonance imaging (MRI) demonstrated a widened thecal sac from L4 to S1, with an extensive scalloping of the laminae and a spinous process caused by multiple thecal diverticula (Fig. 2A). Axial T2-weighted images showed a clumping of the nerve roots of the cauda equina on the side of the ecstatic thecal sac suggesting an adhesion of nerve roots to the ararchnoid membrane. The conus medullaris terminated at the level of L1 body, however, the filum terminale seemed to be adherent to the posterior wall of the thecal sac at L1 level (Fig. 2). No abnormal enhancement or mass lesion such as herniated lumbar disc was found.
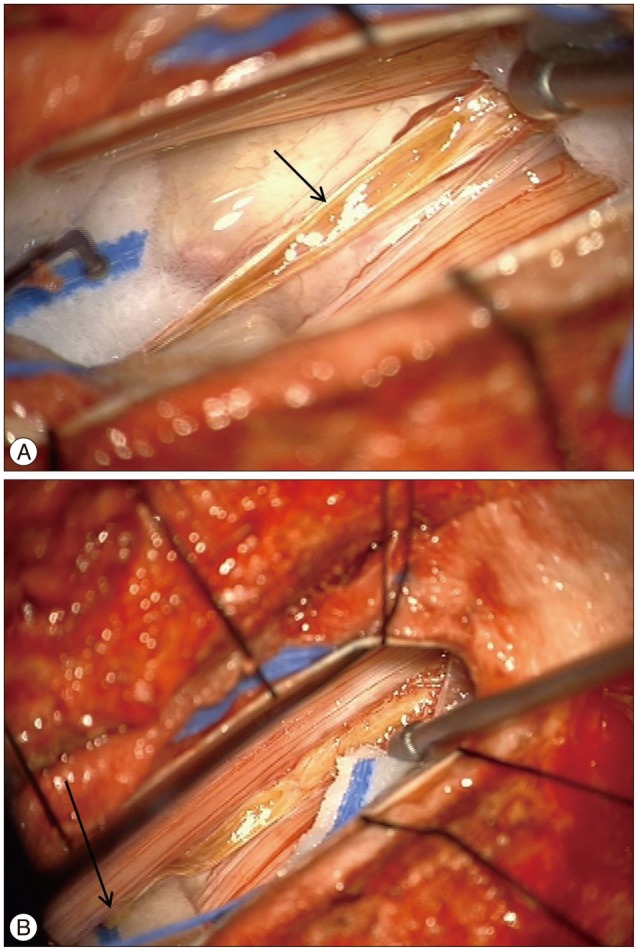
Considering the chronic progressive course of a CES and the radiologic findings of adherence of filum terminale to the posterior wall of the dural ectasia, dethetering of the filum terminale was performed after getting informed consent from the patient and his relatives.
The dura was opened through a laminectomy of L2 under general anesthesia. The filum terminale was identified under microscopic vision and tested with a low current bipolar stimulation (Fig. 3). After identifying the filum, it was coagulated and sharply divided, and the dura and overlying wound were closed in layers. No adverse effect of the untethering procedure was observed and the postoperative course was uneventful as well. After 6 months of follow up, the urinary incontinence was not improved. The left buttock pain was improved; however, the dysesthetic pain and paresthesia in his left posterior calf and sole were not improved.
DISCUSSION
AS may be completely asymptomatic and diagnosed when a radiograph is performed for some unrelated reason. It may also re-present after a period of quiescence, or rarely present late with complications. The rate of neurologic complication of AS was reported to be approximately 2.1%2).
CES is one such rare complication. First described by Bowie and Glasgow5), it occurs in a longstanding disease, with usually more than 15 years duration when this disease is inactive and the patient typically has been symptom-free for many years20). Symptoms are usually sensory in nature, affecting lumbosacral roots and frequently include urinary and bowel symptoms that are usually mild, if present. Despite its relative rarity, it is a condition which has been well described, particularly with imaging techniques of CT and MRI1,18).
The exact pathogenesis of dural ectasis and dural diverticua in longstanding AS is still unclear. Under normal conditions, the meninges and the dural sac absorb the CSF and dampen the transmitted CSF pressure. In the longstanding AS, the process of inflammation may extend to the adjacent dura mater from the paraspinal ligament, resulting in an adhesion of the dura mater to the surrounding structures that reduce the compliance and elasticity of the lower dural sac and its ability to dampen the fluctuation in CSF pressure6,9,13). Thus, an inflamed dura may be weakened, further encouraging the development of dural ectasia and bony erosion12).
Several hypothetical mechanisms have been proposed for CES associated with AS (CES-AS syndrome), including small vessel angitis involving the vasa vasorum of the nerve roots, nerve root damage due to dural ectasia with decreased elasticity of the dural sac and increased arterial pulsatile forces transmitted through the CSF, chronic inflammatory process, and previous spinal irradiation due to treatment of AS6,12,13,17). Of the proposed mechanisms, a chronic inflammatory theory of CES-AS syndrome seems to be most plausible considering the chronic progressive clinical course, the MRI findings of tethering of the conus medullaris and the adhesions of the nerve roots to the dorsal aspect of the dural sac and the spinal canal, as well as the development of a CES-AS syndrome between 17 and 53 years (average, 35 years) after the onset of AS4,12,15). However, the exact prevalence of dural ectasia in AS is still unknown yet.
There is no effective medical or surgical treatment for a dural ectasia, presumably an end result of the chronic inflammatory process2,12). According to a meta-analysis on the treatment efficacy of CES-AS syndrome by Ahn et al.2), steroids were not effective in the chronic stage, while non-steroidal anti-inflammatory drugs decreased the back pain but did not improve the neurological deficit, probably owing to a non-active inflammation in the chronic stage of a CES-AS syndrome. Cornec et al.7) reported a case of dramatic clinical improvement in the anal sphincter function and sensation at anus and buttock with infliximab, a monoclonal antibody to tumor necrosis factor α as a major pro-inflammatory cytokine used in the treatment of active AS.
The effectiveness of surgical treatment for CES-AS is still controversial. There were reports6,8,10,11,16) regarding surgical interventions, such as lumboperitoneal shunting or laminectomy that may improve or arrest the progress of pain, sensory, motor, bowel and bladder dysfunction in few patients based on the theory of attributing nerve root damage to excessive pulse pressure in CSF and to nerve root compression from the dural diverticula. However, most reports2,12), including our case, reported only limited or no clinical improvement after surgical treatment. As an explanation for the absence of improvement after surgical treatment, Arslanoglu and Aygun3) proposed that an initial inflammatory process results in an adhesion of the nerve roots of the cauda equina to the dural sac, and the nerve roots are progressively stretched by the enlarging diverticula. Therefore, Liu et al.12) suggested that the effect of medical and surgical treatment may be determined on the timing of the treatment, whether there is active inflammation or irreversible nerve root damage has developed and the MRI finding and CSF findings showing the status of acute or chornic inflammation may provide valuable information in the treatment planning in a patient with CES-AS syndrome.
CONCLUSION
A CES associated with dural ectasia is a rare, but serious problem in the management of patients with longstanding AS. Although the effectiveness of various medical and surgical treatments is still not established, the prompt identification of this problem with a timely surgical intervention seems to be a reasonable approach in this condition.