INTRODUCTION
Hemangioblastomas (HBs) are highly vascular benign tumors composed of neoplastic stromal cells with rich capillary networks31). They develop sporadically in 67% of all cases and in 33% of cases as a feature of von Hippel-Lindau (VHL) disease22,23,31). HBs may arise in any part of the central nervous system, but are found most frequently in the posterior fossa or spinal cord. HBs in a supratentorial location are rare and have been found in approximately 130 patients, but very limited information on their natural history, incidence, clinical effects, and optimal management is available21). Among these patients, 60% received a diagnosis of VHL disease, whereas infratentorial HB occurs in only 33% of patients with VHL19). Furthermore, supratentorial HB with meningeal involvement is extremely rare.
This case report describes the case of a supratentorial meningeal HB mimicking angioblastic meningioma detected 5 years after surgical resection of an infratentorial HB in a patient with VHL disease.
CASE REPORT
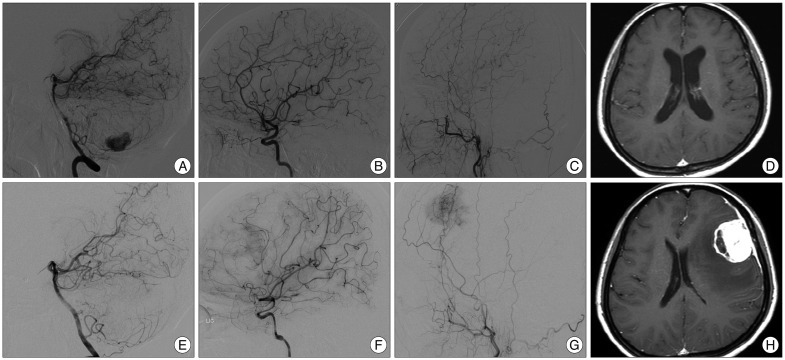
A 51-year-old female patient presented to our hospital with severe dizziness and right facial paresthesia. Five years previously, magnetic resonance imaging (MRI) had shown 2 small masses on the right cerebellum and 1 large mass on the left cerebellum. At that time, lateral vertebral artery digital subtraction angiography (DSA) had shown a highly vascular tumor nodule supplied by the posterior inferior cerebellar artery. Lateral internal carotid artery (ICA) and external carotid artery (ECA) DSA had shown no vascular abnormalities. T1-weighted gadolinium enhancement MRI had shown no abnormal findings in the supratentorial region (Fig. 1A-D). The patient had undergone craniotomy, and the left cerebellar tumor was removed. The pathological diagnosis was HB. Abdominal computed tomography, an evaluation of the patient's family history, and genetic screening had been employed to evaluate the presence of VHL disease. Computed tomography had revealed cysts in the pancreas, liver, and both kidneys. Three of the patient's 6 siblings and 1 of her 2 children were diagnosed with VHL disease. Genetic analysis had shown the presence of a VHL gene mutation; thus, the patient's condition had been diagnosed as HB associated with VHL disease.
Follow-up MRI performed 1 and 12 months after the first operation had shown no evidence of recurrence or abnormal findings in the supratentorial region. However, MRI performed 5 years after the first operation showed a 4.0×3.6×4.0-cm cystic mass with strong enhancement, dural base thickening, and peritumoral edema on the left frontal surface. However, no tumor recurrence was observed in the cerebellum. Left ICA DSA showed pial blood supply to the rim of the tumor. Left ECA DSA showed tumor staining from the middle meningeal artery (Fig. 1E-H).
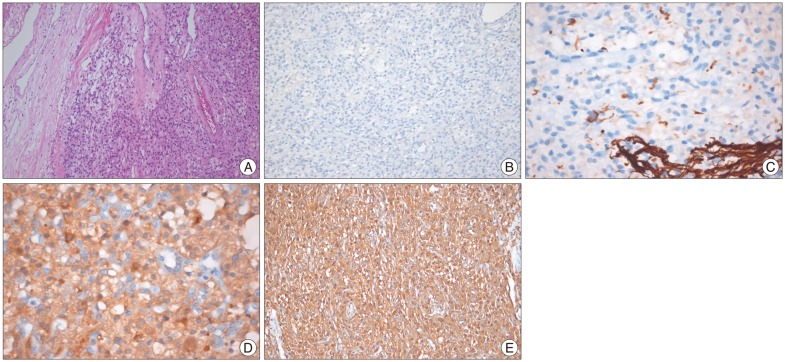
The tumor was completely removed after preoperative embolization. It was a vascular tumor with extensive dural attachment. Histopathological examination with hematoxylin and eosin staining showed lipid-containing vacuoles and abundant vascular cells (Fig. 2A). The tumor cells were negative for epithelial membrane antigen (EMA), glial fibrillary acidic protein (GFAP), CD34, and S100, but positive for neuron-specific enolase (NSE) and vimentin (Fig. 2B-E). The patient was definitively diagnosed with HB with meningeal involvement. The patient's dizziness resolved postoperatively. There was no recurrence of the supratentorial tumor or change in the size of the multiple small right cerebellar tumors during the 12-month follow-up period.
DISCUSSION
HBs are benign tumors of vascular origin that develop in the central nervous system. They account for 2% of all intracranial tumors and 5-15% of posterior fossa tumors in adults4,17). HBs primarily arise in the cerebellum and are rarely found in the supratentorial region.
Supratentorial HB was first described by Bielschowsky in 190226). Approximately 130 cases of supratentorial HB have been reported. HB tumors are frequently found in the cerebrum and sellar/suprasellar and intraventricular regions. In order of frequency, they are found in the frontal, parietal, and temporal lobes of the cerebrum, and there are a few reports of congenital HB11,24). Among patients with supratentorial HB, 60% were diagnosed with VHL disease, and this condition is more common in VHL disease than infratentorial HB (33%)22,23,31). A significant association was found between HB tumors located in the sellar/suprasellar region and a diagnosis of VHL disease19). HBs may present as either isolated or multiple lesions. Most reported HBs were solid tumors, whereas one-third were found to be cystic.
However, meningeal HBs in a supratentorial location are extremely rare. Only 16 patients with meningeal involvement of HB have been described in the literature (Table 1). The sex ratio is equal, and the patient age range is 10-72 years. Tumors are mostly located in the frontal lobe, but they are also found in the parietal lobe. All these HBs had a solid consistency, and only 2 were associated with VHL disease, including the case reported here (12.5%). Four patients had multiple lesions and 3 had infratentorial HB, 2 of whom had VHL disease.
Ammerman et al.2) reported that "Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease", excluding those in a supratentorial location, had a stuttering or stepwise growth pattern. In addition, approximately 45% of symptomatic HBs were not visible on the initial MRI. Wanebo et al.32) reported that 44% of infratentorial and spinal cord HBs seemed to grow during the follow-up period. In addition, Peyre et al.21) reported that "Natural history of supratentorial hemangioblastomas in von Hippel-Lindau disease", of 11 supratentorial HBs followed-up with serial MRI for at least 6 months, 10 enlarged and 1 remained stable. Enlarged tumors showed either continuous (n=7) or intermittent (n=3) growth patterns. Furthermore, 7 tumors were followed-up for more than 24 months. Five of these tumors showed a rapid increase in size after 24 months. Thus, supratentorial HBs (92.9%) show a higher incidence of tumor growth during follow-up than infratentorial and spinal cord HBs (44%). Nevertheless, the natural history of supratentorial meningeal HBs is unclear because it is extremely rare.
It is difficult to make a diagnosis of supratentorial meningeal HB by preoperative neuroimaging or microscopic observation of the tumor during surgery. The main differential diagnoses of supratentorial meningeal HB include angioblastic meningioma, hemangiopericytoma, and metastatic renal cell carcinoma. The HBs show variable cellularity and consist of 2 major components (vascular channels and stromal cells) that stain negative for epithelial markers such as EMA and vascular markers such as CD34. Therefore, EMA immunostaining can especially help distinguish supratentorial meningeal HB from meningioma with the former being negative for EMA staining20). In addition, CD34 immunochemical activity is positive in some cases of HB but shows more focal, heterogeneous, weak staining of tumor cells in meningioma and hemangiopericytoma7,10,29). Furthermore, HB tumors are generally negative for GFAP and positive for NSE. Additionally, S-100 protein showed a variable immunoreactivity among neuroectodermal markers3,9,30). Finally, vimentin, the most widely distributed intermediate filament expressed in virtually all mesenchyma and corresponding tumors, is positive in HB tumors, supporting the hypothesis that HBs originate from the mesenchyme. In our case, the tumor was negative for EMA, but positive for NSE and vimentin. Recently, inhibin-α has been described as a useful marker for distinguishing HB from angiomatous meningioma28). In addition, histologically, HB may be difficult to distinguish from renal cell carcinoma because both tumors are highly vascular and contain cells with pale cytoplasm. Immunohistochemically, renal cell carcinomas stain positive for EMA whereas HB are EMA negative6).
The treatment of choice for supratentorial meningeal HB is surgical resection. Mills et al.19) reported that patients with supratentorial HB undergoing gross total tumor resection (GTR) experienced a significant improvement in progression-free survival (PFS) compared with those receiving subtotal resection (STR) (5-year PFS : GTR, 100% vs. STR, 53%). However, treatment modality and the literature are not definitive because of the rarity of supratentorial meningeal HBs. In our opinion, total resection of the tumor prevents recurrence and postoperative hemorrhage and radiotherapy may be an alternative or adjuvant therapy for multiple, subtotally resected, and recurrent tumors.
CONCLUSION
We reported a rare case of supratentorial meningeal HB that was detected 5 years after surgery for cerebellar HB in a patient with VHL disease. Although supratentorial meningeal HBs are extremely rare tumors, differential diagnosis of meningioma and other meningeal-based tumors should be investigated. Pathological and immunohistochemical techniques are helpful for such diagnoses. Definitive treatment for these lesions is surgical resection. Accordingly, physician should be aware that supratentorial meningeal HBs can be developed in patient with VHL disease and regular follow-up are mandatory.