INTRODUCTION
Stroke is the third most common cause of death globally after coronary heart disease and cancer. The incidence of stroke and the number of related deaths are increasing because of the increased percentage of the elderly in the population. Stroke also causes disability and significant socioeconomic damage in the population. It is the cause of neurological damage and many psychiatric and systemic complications such as depression and anxiety. The financial damage caused by decreased productivity in addition to the treatment costs of the disease and rehabilitation period are quite significant23). The psychological damage that develops after stroke is another aspect of the event and delays healing. Many drugs that show their effect through various mechanisms and various surgical methods have therefore been tried to prevent the ischemic damage caused by the primary ischemic or traumatic cerebrovascular events or the secondary injury. However, there is still no method or drug that can completely solve of this problem. Many of the drugs that have been tested are effective on one or several mechanisms but this chain of complicated and multi-factorial events cannot be broken and there is only a limited effect56).
Ischemia-related apoptotic death and the penumbra area have recently become more popular subjects and are now the focal point of many studies. Several articles emphasized the relationship between 5-hydroxytryptamine-2 (5-HT2) agonist and antagonists and apoptotic cell death16,17,27,49,55,65). We therefore planned our study believing that quetiapine, a dibenzothiazepine derivative atypical antipsychotic drug, could decrease apoptotic cell death and the negative effects of secondary injury in cerebral ischemia while also preventing the neuropsychiatric complications caused by this ischemia.
MATERIALS AND METHODS
This study was performed on a total of 30 rats in 5 experimental groups each containing 6 randomly selected rats obtained from the Gazi University Experimental Animals Research Center (GUDAM). The groups were as K-I, the control group where no surgical procedure was performed (n=6); K-II, the ischemia control group that lived for 1 day (n=6); K-III, the ischemia control group that lived for 3 days (n=6); D-I, the ischemia-medication group that lived for 1 day (n=6); and D-II, the ischemia-medication group that lived for 3 days (n=6). The K-I group was sacrificed to compare the apoptosis in the brain of healthy rats with those that had undergone ischemia. We performed the same surgical procedures in the other 4 groups to create ischemia and to sacrifice the animals. Taking the result of previous studies into account, the medication groups received quetiapine 10 mg/kg/day intraperitoneally 30 minutes after the trauma we caused, and then at the same time every day for 3 days24,51).
Animals and ischemia surgery
Male Wistar type albino rats (n : 30) weighing 200-220 gr were kept in a room at 21ôÝ1ã temperature at 12 hours darkness and 12 hours light with 4 rates in a cage and ad lib water and feed.
General anesthesia was administered intramuscularly by using 10 mg/kg xylazine (Rompun, Bayer, Istanbul, Turkey) and 50 mg/kg ketamine hydrochloride (Ketalar, Eczacibasi, Istanbul, Turkey). Anesthesia depth was evaluated with a painful stimulus through the tail every 15 minutes. All surgical procedures were performed under the microscope (Opmi 99, Carl Zeiss, Germany).
Cerebral blood flow (CBF) monitorization was with a laser Doppler probe (Vasamedics, Blood Perfusion Monitor, Model BPM 433-1, St Paul, MN, USA) after a burr-hole was opened in the left middle fossa using a high-speed drill (Aesculap Microtron GD 412, Tuttlingen, Germany). After the 0 minute measurement, the left carotid artery bifurcation was dissected with a ventral midline neck incision for the MCA occlusion procedure. Transient focal cerebral ischemia was created using the MCA intraluminal filament method defined by Kawamura et al.37) in 1991. We observed decreased blood flow on the CBF monitor when full occlusion was achieved. We accepted that the suture closed the ostium when the 0 minute CBF measurement had decreased at least 80%. All surgical procedures took 25 minutes on average. Reperfusion was achieved by removing the suture under anesthesia after 120 minutes of occlusion.
Animals in the K-II and D-I groups were sacrificed on the 24th hour of trauma. Animals in the K-III and D-II groups were sacrificed after being followed-up for 3 days. The K-I group, where no surgical intervention causing ischemia had taken place or medication had been given, was sacrificed to use as a comparison for apoptosis in healthy brain tissue.
Immunohistochemical study
All the histological and immunohistochemistry experimental studies were performed at the Hacettepe University Histology and Embryology Department laboratories.
The total brain tissue obtained from the rats was fixed with 10% buffer formaldehyde for 3 days. The dehydration and paraffinization procedures were performed with a tissue processing device (Leica TP 1020 CH, Germany). The paraffin embedding and block formation procedures were performed on the paraffin station (Leica EG 1150 CH, Germany).
Sections 5 ôçm thick were obtained from the paraffin blocks on special silanized slides. Following deparaffinization with heat for 1 hour once and chemical deparaffinization twice with xylol for 15 minutes, they were stained with hematoxylin-eosin and the accuracy of the field was checked. Other sections were marked with the immunohistochemical TUNEL method using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon, USA, cat no : 7101). Background staining was provided by keeping the specimens in 0.5% methyl green for 10 minutes. Differentiation was with 100% n-butanol. We finally applied closure balsam and covered the material with a coverslip.
Evaluation of sections
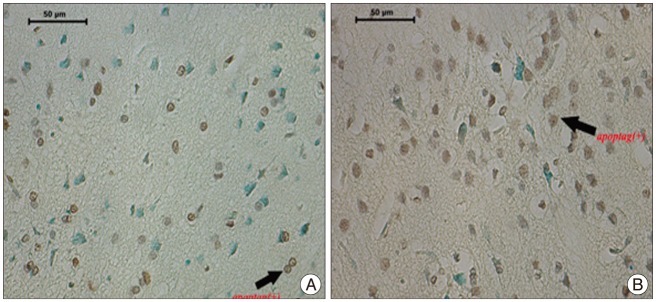
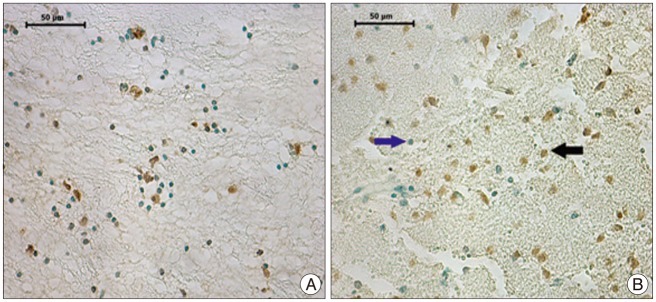
The immunohistochemically stained sections of the group were evaluated on the light microscope (Leica CTR 6000, Germany). Nuclei stained with Methyl Green were evaluated as normal while cells with brown nuclear staining were considered ApopTag positive. Sections for all animals in each group were evaluated under 40û magnification. Five random areas that showed the most intensive ischemia were photographed (Leica DFC 490 camera, Germany) and the ApopTag positive cells were counted in each field. The apoptotic cells of the groups are shown in Fig. 1, 2.
RESULTS
Table 1 presents the data on ApopTag positive cells stained brown on a pale green background that were seen with the evaluation of 5 different randomly selected areas from the ischemia region for each animal in the groups (Table 1, Fig. 3).
Table 1 shows that the number of apoptotic cells increases significantly in the control group with time (in the K-III group compared with the K-II group). This time-related marked apoptotic cell increase is not seen in the medication group (between the D-I and D-II groups). The 1-day results show less apoptosis in the D-I group than the K-II group but with no statistical significance. The 3-day results show that the D-II group had markedly less apoptosis than the K-III group and that this is statistically significant.
Statistical analysis
Statistical analysis results are shown in Table 1. When all the groups were evaluated the p value was <0.001 indicating a significant difference between the groups for the number of apoptotic cells. The control group where no ischemic procedure was performed (K-I) was found to be statistically significantly different when compared with two-way comparisons with the other two groups that indicate that the method used for ischemia was successful (p=0.002). We did not find a significant difference when comparing the K-II, D-I, and D-II groups (D-I/D-II : p=1.000, K-II/D-I : p=0.699, K-II/D-II : p=0.699). The K-III group had the largest number of apoptotic cells and we found a statistically significant difference when we compared it with the other groups (K-III/D-I : p=0.002, K-III/D-II : 0.004, K-II/K-III : p=0.004). There was a statistically significant difference between the K-II and K-III groups but none between the D-I and D-II groups indicating that the medication treatment was effective.
DISCUSSION
Stroke can lead to many neurological, psychiatric and systemic complications and has social and socioeconomic significance. It can develop as a result of ischemia, bleeding, or secondary injury. The central part of the ischemic area following stroke develops irreversible necrosis within minutes while the cell integrity is not yet structurally damaged in the penumbra area and the reestablishment of cerebral blood flow and use of neuronal protective agents can prevent ischemic injury in this area as the ion pumps are still healthy enough to continue their function, thus making this a target area. Energy-dependent apoptosis is seen especially in this penumbra area10,50,56,72).
Apoptosis has first been used by the Scottish investigators Kerr et al.38) in 1972 to mean "fall separately". Apoptosis is currently defined as cell suicide and is a type of cell death where the organism can eliminate cells that have completed their biological function or are injured without damaging the surrounding cells, and it also plays a role in keeping the number of cells in tissues under control. It is effective in both pathological and physiological events, is genetically controlled and consists of programmed biochemical and morphological changes. Apoptosis is an active function and requires high ATP levels7,15,26,32,36,40,44,50,56,58).
Recent studies have selected the penumbra area that shows apoptosis as the target tissue that can be saved9,56). Experimental studies have aimed to decrease or slow down this apoptotic process to gain time and ensure that some functions are regained with reperfusion. Despite this positive clinical picture and the possibility to recover some functions with reperfusion, the damage can also increase with the formation of free oxygen radicals21,56).
Several recent studies with glutamate receptor antagonists20,43), calcium channel blockers25), free oxygen radical eliminating agents21,60), citicholine1-4,56), hypothermia5,47) and sometimes a combination of these agents have provided positive neuron protective results.
Experimental and clinical cerebral ischemia studies have shown that moderate hypothermia (2-6ã decrease) has a neuron protective effect by itself or when used in combination and is well tolerated55,56). Although the mechanism of action has not been fully elucidated, it is thought to show its effect by decreasing the metabolic rate, protein synthesis and ATP usage, preventing the formation of free oxygen radicals and blocking the release of excitatory neurotransmitters41,42). It has been reported that particularly positive results can be obtained with mutual reinforcement when it is used together with neuron protective substances5,56).
Citicholine (CDP-choline), a necessary nucleoside for phospholipid synthesis and repair that play a role in the continuity in the cells membranes, has been shown to decrease the DNA fragmentation that appears following ischemia and the pro-caspase 1, 2, 3, 6 and 8 expression that plays a role in the apoptotic process2,4,47,56,59,67). Some reports indicate that, when combined with hypothermia, it can prevent the apoptotic cell death stages without the creation of a apoptosome, and can decrease excitatory amino acid (EAA) accumulation and therefore neuronal damage that develops following a lack of energy45,56,59,69).
An experimental study by Luo and Shi46) has shown that polyethylene glycol can decrease apoptotic cell death following spinal trauma by suppressing cytochrome C release, one of the most important steps of apoptosis. The preventive effect against apoptosis in the posttraumatic spinal cord has been shown for 17-beta estradiol by Yune et al.73), for erythropoietin by Arishima et al.8) and Vitellaro-Zuccarello et al.64) and for antioxidants by Bao and Liu11,12).
Gupta et al.30), in their 2002 study, have shown that adenosine, and endogenous inhibitory neuromodulator in the central nervous system, decreases the release of EAA secretion during ischemia and protects the neurons against oxidative stress.
Caserta et al.19) have found that the irreversible general caspase inhibitor QVD-OPH prevents Caspase 8 and 10 activation related to the Fas/CD95 death receptors and Caspase 9 and 3 related to cytochrome C, thus preventing apoptosis in both neuronal and glial cells, decreasing secondary injury and contributing to functional recovery.
Studies by Muto et al.49) in 2005 and Rajesh et al.55) in 2006 have shown that Sarpogrelate, a 5-HT2 receptor blocker, prevents the apoptotic death of cells in the cardiac tissue with its antiapoptotic effect and also shows a protective effect against myocardial dysfunction. Capela et al.16,17) have studied ecstasy (MDMA) and shown that it causes apoptotic cell death through the 5-HT2A receptor pathways and that this apoptosis can be prevented using the 5-HT2 blocker agent Ketanserine. Tascedda et al.63) in 1999 and Tarazi et al.62) in 2003 have shown a neuroprotective effect of quetiapine through NMDA receptors with chronic usage.
We planned our study taking these articles on 5-HT2 agonists and antagonists into account and used the dibenzothiazepine derivative atypical antipsychotic drug quetiapine believing that it may prevent apoptotic cell death and decrease the secondary damage caused by trauma in cerebral ischemia. The statistical results of our study show that comparison of K-I with the other groups has shown a significance of p=0.002 indicating that the ischemic procedure used to create apoptosis was successful. There was also a significant difference between the 1st day and 3rd day control groups (K-II/K-III : p=0.004), indicating increasing apoptotic cell death with time. Statistically, there was no increased apoptotic cell death with time in the groups administered medication (D-I/D-II : p=1.00). Our main purpose was to show whether our medication was effective and there was no statistically significant difference between the medication group on day 1 and the control group (K-II/D-I : p=0.699) while a statistical difference was present between the medication group on day 3 and the control group (K-III/D-II : 0.004). All these results have demonstrated immunohistochemically that quetiapine decreased time-related apoptotic cell death.
Yan et al.70,71) have reported that global cerebral ischemia causes hypocampal neurodegeneration, neuropsychiatric disorders and memory loss and that quetiapine can prevent these developments with its neuroprotective effect18). Administering quetiapine before ischemia has also been found to markedly decrease depression and anxiety-type behavioral changes triggered with global cerebral ischemia in mice18). The use of quetiapine and olanzapine has been shown to have a significant effect on decreasing PC12 cell death and this cytoprotective effect is regulated through the translocation and expression of proapoptotic bax and antiapoptotic Bcl-XL or through the increase of the antioxidant enzyme superoxide dismutase-153,66,71).
Another mechanism in ischemic events is the glutamate-related excitoxicity. It is thought that ischemia and oxidative stress increase glutamide-related excitotoxicity and neuronal injury28) and that quetiapine shows a neuroprotective effect by regulating glutamate receptor expression in the hypocampus and other cerebral regions and also decreases schizophrenia symptoms through this system54,62,63).
Various studies have shown that atypical antipsychotic drugs including quetiapine prevent the apoptosis caused by N-methyl-4-phenylpyridinium ions53) while another study has reported that they block the caspase-3 activation caused by beta-amyloid peptide and decrease apoptosis63). Atypical antipsychotics including quetiapine have been shown to increase antiapoptotic Bcl-2 but Jarskog et al.35) have found that subchronic antipsychotic usage increases caspase-3 activity without increasing DNA fragmentation and that it does not support Bcl-2 mediated neuroprotective efficacy despite what is currently believed.
Motor, sensory, visual, and speech disturbances are dominant among the clinical problems caused by microscopic cerebral ischemia. Some neuropsychiatric diseases, with depression being the most common, can be encountered in the early and late periods but may be missed or not emphasized due to the severe neurological picture of the patient. These conditions can also limit physical and cognitive recovery13,14,22,68,71). From this point of view, apoptotic cell death, hypocampal neurodegeneration and effect of atypical antipsychotics on these disorders and especially schizophrenia have become popular study subjects in ischemia.
Most of the medications and methods used for neuroprotection and to prevent ischemia-related problems are not related to clinical use despite their beneficial effects. The quetiapine that we used in our study had marked efficacy especially when we look at our 3rd day results. It is also currently in use for schizophrenia, and unipolar18,48) and bipolar depression15,18) and is an effective medication with a better side effect profile than antipsychotics. It is also safe regarding extrapyramidal side effects6,33,52,61). Clinical studies have revealed that quetiapine is also a suitable agent for the treatment of aggression and agitation due to traumatic cerebral damage39). Quetiapine causes blockage with its high binding affinity for serotonin 5-HT2 and 5-HT6 receptors and the more moderate affinity for dopamine D2 receptors. It also has affinity towards öÝ1 and öÝ2 receptors and H1 receptors like clozapine. It shows negligible binding to dopamine D1 and Muscarinic M1 receptors33,34,57,61). Although it possesses clozapine-like antipsychotic effects, its lower affinity for muscarinic and dopaminergic receptors ensures less anticholinergic and extrapyramidal system side effects and less effect on prolactin levels29,31). Some studies suggest a wider clinical use due to its many neuroprotective and neuropsychological effects as mentioned in our study18). Both our results and the fact that quetiapine is already used clinically also support a wider clinical perspective for this medication. However, further clinical studies are needed to support the efficacy of quetiapine in ischemia. Despite all these beneficial features, the US Food and Drug Administration has reported possible increased mortality with the use of atypical antipsychotic medications in the elderly with cardiovascular and infection problems in addition to the dementia-related psychosis18).
Studies mostly performed by psychiatry departments have emphasized many different mechanisms while studying the effect of ischemia and apoptosis in neuropsychiatric disorders and the mechanism of action of atypical antipsychotics in these disorders. In conclusion, quetiapine has been shown to decrease ischemia-related apoptosis in both our study and other studies.
CONCLUSION
Our results implicate that quetiapine which is emphesized effectiveness of prevention in neuropsychtric diseases and enlarge its usage area by pyschiatrists, also should be discussed by neurologists and neurosurgeons study on ischemia, be taken part in studies on clinical reflection of reducing apoptosis after ischemia, and remember its beneficial effect on overlooked ischemic neuropsychatric problems.