Ha, Gwak, Rhee, Youn, Choi, and Cheon: Intracavitary Radiation Therapy for Recurrent Cystic Brain Tumors with Holmium-166-Chico : A Pilot Study
Abstract
Objective
Intracavitary injection of beta-emitting radiation source for control of cystic tumors has been tried with a benefit of localized internal radiation. The authors treated cystic brain tumor patients with Holmium-166-chitosan complex (Ho-166-chico), composed of a beta-emitting radionuclide Holmium-166 and biodegradable chit polymer, and evaluated the safety and effective measurement for response.
Methods
Twenty-two patients with recurrent cystic brain tumor and/or located in a deep or eloquent area were enrolled in this pilot study. The cyst volume and wall thickness were determined on CT or MRI to assess radiological response. The activity of Ho-166-chico injected via Ommaya reservoir was prescribed to be 10-25 Gy to the cyst wall in a depth of 4 mm.
Results
There was neither complications related to systemic absorption nor leakage of Ho-166-chico in all 22 patients. But, two cases of oculomotor paresis were observed in patients with recurrent craniopharyngioma. Radiological response was seen in 14 of 20 available follow-up images (70%). Seven patients of 'evident' radiological response experienced more than 25% decrease of both cyst volume and wall thickness. Another 7 patients with 'suggestive' response showed decrease of cyst volume without definitive change of the wall thickness or vice versa. All patients with benign tumors or low grade gliomas experienced symptomatic improvement.
Conclusion
Ho-166-chico intracavitary radiation therapy for cystic tumor is a safe method of palliation without serious complications. The determination of both minimal effective dosage and time interval of repeated injection through phase 1 trial could improve the results in the future.
Key Words: Holmium · Chitosan · Intracavitary radiation · Intracranial cyst.
INTRODUCTION
The intracranial cystic tumor poses troublesome problems, especially when it is in a deep location or near to critical neurovascular structures. Percutaneous aspiration of the intracranial cyst has been tried via the Ommaya reservoir system 3,4,21), but the aspiration itself provides only a temporary relief of symptoms rather than a therapeutic effect. After the first attempt of intracavitary radiation for cystic craniopharyngioma in 1950 by Leksell and Liden 13), a variety of beta and gamma emitting radioisotopes have been tried as internal radiation sources for cystic brain tumors ( 32P, 90Y, 198Au) 9,19,22,25). Although they were delivered in colloidal form or microsphere to prevent systemic absorption, considerable amount of intracavitarily infused isotope was systemically absorbed even in cases not causing obvious systemic complication 2,15). The Korean Atomic Energy Research Institute (KAERI) developed a Holmium-166-chitosan complex (Ho-166-chico) to solve the problem of systemic absorption 18). Holmium-166 has features of an ideal radioisotope for internal radiation therapy including a suitable half-life (26.8 hours), high beta energy and low-dose gamma ray which was useful in imaging or monitoring 11). In pre-clinical study, the intratumoral distribution and cytotoxic effect of beta emission from unsealed Holmium-166 was proven in animal models of various solid cancers 11,17). But, this free form of Holmium-166 caused considerable systemic absorption and was distributed to liver, spleen, lungs and bones 24). To retain radioactivity mostly at the target site and prevent systemic absorption, chitosan, a polymer of 2-deoxy-2-amino-D-glucose was introduced. Chitosan, which is obtained by deacetylation of natural chitin, is biocompatible, bioaffinitive, and biodegradable 16). It forms a chelate with the heavy metals and is readily dissolved in water to make a clear solution under acidic conditions (optimal at pH 3.0) at room temperature 18). Suzuki et al. 24) proved that 166Holmium causes hardly any systemic absorption when it forms a chelate complex with chitosan in animal experiment. In their experiments, intrahepatically injected Ho-166-chico was localized at the administration site (>90% of radioactivity) after 144 hours with less than 1% of urinary and fecal excretion. The authors obtained a pure solution of a Ho-166-chico for injection at the facility of KAERI. For effective control of cystic brain tumors by enhanced radiation delivery to the tumor wall, the authors tried intracavitary injection of Ho-166-chico via stereotactically installed Ommaya reservoir in 22 selected patients with cystic brain tumors. As a pilot study, a proportion of Ho-166-chico bound to the cyst wall was calculated by measuring residual radio-activity of cystic fluid and possible systemic absorption was checked in urine and blood samples in selected patients. We newly defined the radiological response of cystic brain tumors considering not only cyst volume but also wall thickness and its clinical usefulness is worth reporting as a pioneering study of Ho-166-chico for cystic brain tumor.
MATERIALS AND METHODS
Eligibility criteria
From April 1996 to December 2004, thirty-four injections of Ho-166-Chico were administered to 22 selected patients with intracranial cystic tumors. All tumors were histologically proven before isotope injection, and patients had received some prior treatment, except two metastatic tumor patients, in whom the histological diagnosis was made at the time of stereotactic Ommaya reservoir insertion. The selection criteria were as one of follows : 1) located in deep or eloquent area; 2) having high risk for re-operation of recurrent tumor or already received conventional radiation therapy; 3) immediate aspiration of the cyst via Ommaya reservoir is necessary for the relief of critical symptom. All patients agreed to this pilot study with informed consent. This pilot study obeys all the rules and regulations of clinical study regarding human subject protection and received Institutional Review Board approval [KIRAMS-04-12(1)].
Preparation of Ho-166-chico
Holmium-166 was obtained by bombarding Holmium-165 in a nuclear reactor (KAERI, Daejeon, Korea) as a solution of 166Ho-(NO3)3·5H2O. The specific activity of the solution was measured immediately after irradiation. Chitosan was prepared by Pharmaceutical Development Laboratory (Dong Wha Pharm. Ind. Co., Ltd., Anyang, Korea) and lyophilized before use by the evaporation of acetic acid used as the solvent. To facilitate the formation of complexes of holmium and chitosan, ascorbic acid was added to chitosan solution before freeze-drying. On the day of use, Ho-166-chico was prepared by mixing the holmium and chitosan solutions. Thereafter, vials were shaken vigorously for 2-3 min using a vortex mixer and left at room temperature for 30 min. The radioactivity of Ho-166-chico was measured just before the injection and diluted to a designated dose of 1 mL volume with normal saline.
Ommaya installation and leakage test
An Ommaya reservoir (Baxter Healthcare Corporation, Deerfield, IL, USA) was installed using BRW stereotactic system prior to Ho-166-chico injection in all cases. The purposes of installation were 1) exact and repeatable injection of the isotope; 2) aspiration of cystic fluid for immediate symptom relief, whenever it is needed; 3) pre-injection leakage test via the reservoir. The reservoir was installed usually three weeks prior to injection of the isotope to prevent the possible leakage along the puncture tract. The leakage test was performed using CT scan after an injection of 1 cc of diluted metrizamide solution or Omnipaque-300 ( Fig. 1). It was useful to detect any possible minor leakage and to measure the exact cyst volume just before the injection. The pre-injection CT or MRI was reviewed to measure cyst volume and maximum wall thickness using Image tool software (version 2.0, alpha 3, University of Texas Health Science Center in San Antonio). The mean tumor diameter was deducted from measured volume under assumption of a complete sphere to as a parameter for further dose simulation.
Dose simulation and injection
The dose simulation was performed under the assumption that the cyst was spherical in shape and the isotope was evenly distributed in the cystic fluid or completely bound to the cyst wall ( Fig. 2). The energy transferred in soft tissue was calculated using EGS4 code, Monte Carlo simulation method presented by Prestwich et al. 20). According to the computer simulation, the cumulative absorbed energy at the cyst wall is approximately 60% of maximal dose at 1 mm and 90% at 2 mm of wall thickness. Activity of Ho-166-chico was higher approximately from 4 times to 20 times in the model of complete bound compared to the activity of complete dispersion according to the cyst diameter ( Fig. 2). Dose selection was done under the mainstream of "minimal dosage with repeated injection" to avoid side effects. Thus, between two assumptions of Ho-166-chico distribution, we chose the assumption of complete bound to yield the amount (mCi) of Ho-166-chico to be delivered.
Monitoring of distribution
The distribution of injected isotope was monitored by gamma camera or 3-head SPECT gamma camera after percutaneous injection of the calculated dose of Ho-166-chico ( Fig. 3). To estimate the proportion of wall bound form, the residual radioactivity of tumor cystic fluid was checked at a designated time (1, 2 and 12 hours). Systemic abruption of Ho-166-chico was monitored through examination of patients' urine and blood samples from 24 hours after the injection and/or Geiger counter on patients' extremities.
Evaluation of clinical response
Patients with 'good' response had almost complete relief of symptoms from the cyst with no need of further cyst aspiration. Patients with a 'fair' response had a partial relief of symptoms and decreased frequency of cyst aspiration. Patient with a 'poor' response had no benefit from the Ho-166-chico injection with aggravation of the symptoms necessitating further therapy or increased frequency of cyst aspiration.
Radiological response
Radiological response was evaluated on follow-up CT or MRI of one and/or three month after the treatment. Although there are no recognized criteria for assessment of radiological response of cystic brain tumors even in up-to-date RANO criteria 26), it seems reasonable to measure both the cyst volume and the wall thickness to evaluate radiological response of cystic brain tumor. Thus, the change of cyst wall thickness was also measured at the thickest portion in addition to the change of cyst volume. 'Evident' radiological response is defined as decrease in both the cyst size and the wall thickness more than 25%. 'Suggestive' radiological response indicates decrease in the cyst size but without definitive change (≤25%) of the wall thickness or decrease in the wall thickness more than 25% without a change of cyst size. The cyst evaluated as 'poor' radiological response, showed increase of the cyst size or the wall thickness of equal or more than 25%. All the other status was defined as 'stable'.
RESULTS
Patients' characteristics
Age at the time of entry was average 40.6 years (range 6-66) ( Table 1). There were eleven each of male and female patients. Seven patients had metastatic tumor cysts. Six of seven metastatic tumor patients had lung cancer as a primary tumor and another patient had a breast cancer. Eight glioma patients were included (4 glioblastoma, 1 anaplastic astrocytoma, 3 low grade astrocytoma). All six treated cystic craniopharyngioma patients recurred after the first operation and did not receive radiation therapy except for one patient already underwent Gamma knife radiosurgery ( Table 2). One recurrent trigeminal schwannoma patient was also treated.
Ho-166-chico treatment
Activity of Ho-166-chico per injection ranged 4-20 mCi ( Table 1). We decided to deliver 10 to 25 Gy at 4 mm from the inner cyst wall while considering the patient's expected survival, degree of histological malignancy, or the existence of neighboring critical structure. The injected dose per volume ranged from 0.17 to 3.95 mCi/mL, as we intended the dose per each injection to be less than 5 mCi/mL. Repeated injection of Ho-166-chico was determined according to the radiological and clinical response at least one month apart. The number of injections ranged from once to 5 times (mean 1.4 per patients).
Monitoring of dose distribution
A gamma camera confirmed intracavitary localization of injected Ho-166-chico without leakage in all patients. Systemic absorption was negligible (<1 µCi) measured by radioactivity of patients' urine and blood samples at 24 hours in 4 selected patients (3 patients with metastasis and one patient with craniopharyngioma) and estimated by Geiger count activity of extremities in the other patients. To estimate proportion of wall bound form, we checked residual cystic fluid radioactivity at 1, 2 and 12 hours after the injection in these patients. We presumed that residual radioactivity of cystic fluid represented the radioactivity of freely dispersed proportion remaining after wall bound and the bound proportion (1-dispersed proportion) was calculated considering emission by time ( Fig. 4). The rate of bound at 2 and 6 hours showed difference according to the tumor type as 90% (±3.8, standard deviation) and 97% (±0.6) in the metastasis, whereas 9.8% and 78% in a craniopharyngioma. However, after 12 hours, 92% of the injected Ho-166-chico was wall bounded in a craniopharyngioma.
Complications
Infection of Ommaya reservoir occurred in one patient and resulted in intracavitary abscess. The reservoir was removed and managed with antibiotics administration (case 6). One case of reservoir malfunction was diagnosed as the cystic portion remained (case 13). Transient deterioration of neurological function was noticed in three patients. A metastatic tumor patient experienced re-aggravation of left hemiparesis 24 hours after the Ho-166-chico injection (case 1). However, his hemiparesis was normalized with steroid treatment in 2 weeks. He received two more session of the Ho-166-chico injection successfully and the radiological response was 'evident' ( Fig. 5). Two craniopharyngioma patients suffered from diplopia due to unilateral third cranial nerve palsy. The palsy was completely resolved in a month in a 6-year-old boy who showed 'evident' radiological response (case 19) ( Fig. 6). However, a 26-year-old female patient, for whom the densest Ho-166-chico was administered, remained with failure of meiosis in spite of recovery of eyeball deviation (case 21). One trigeminal schwannoma patients complained of cerebrospinal fluid (CSF) otorrhea 2 weeks after Ho-166-chico injection, but the CSF leakage was successfully managed with lumbar drainage, and she received one more session of Ho-166-chico therapy after leakage test (case 22).
Overall response rate
Patients were monitored after the treatment from 1 month to 53 months (mean 13.2 months). The pre-injection CT or MRI was reviewed to measure cyst volume and maximum wall thickness. The pre-treatment cyst size ranged from 3.8 mL to 60.0 mL (mean 17.7). The wall thickness in the thickest portion varied according to the nature of cyst, ranged 1 mm to 11 mm ( Table 1). Post-treatment images were obtained in all patients except two patients (case 3 and 6), whose general condition rapidly deteriorated and refused brain image follow-up. In another patient (case 9), who rapidly progressed to brain herniation, the exact size of the cyst could not be measured due to massive edema. Post-treatment cyst volume decreased more than 25% in 12 of 19 patients, thus the response rate in terms of the cyst volume was 63% ( Table 1). Eleven of 20 patients (55%) showed more than 25% decrease of the wall thickness. Seven patients showed the decrease of both the cyst volume and the wall thickness, and categorized to 'evident' radiological response. Four patients revealed definitely reduced wall thickness but their cyst volume remained 'stable'. Another three patients showed decrease of cyst volume without the change of wall thickness. These two groups of patients were defined to show 'suggestive' radiological response. Hence, overall radiological response rate was 70% (14 out of 20 patients). In spite of definite cyst volume reduction, two patients with thin walled cyst (case 4 and 14) were defined to show progression due to increase of wall thickness of shrinked cyst.
Radiological and clinical response according to the tumor histology
Although the number of cases was not enough to analyze statistically, the radiological and clinical response seemed different according to the tumor histology. High grade glioma (4 glioblastoma and 1 anaplastic astrocytoma) groups showed the worst result. All four glioblastoma patients deteriorated within 3 months, though one patient showed a 'suggestive' response at 1 month on CT image (case 10). Three patients with low grade astrocytoma showed excellent response despite the lowest Ho-166-chico dosage (mean=0.27 mCi, range 0.17-0.43 mCi/mL) among histological groups. All three patients experienced clinical improvement of their symptoms maintained up to 5, 10 and 18 months, respectively. Two patients revealed 'suggestive' radiological response, and the other patient evaluated as 'progression' due to severe shrinkage of the cyst resulting in increase of wall thickness from 1 mm to 2 mm (case 14).
The metastatic tumor group showed variable results according to their primary tumor and the general condition of patients. One metastatic breast cancer patient showed 'evident' response and had maintained the response up to 12 month, when she died from massive pulmonary metastasis (case 5). One of six metastatic lung cancer patients failed to get a follow-up image due to her poor clinical condition and died from aspiration pneumonia (case 3). Another patient's lesion progressed on 2 months follow-up image and she died from the progression of the brain lesion in spite of whole brain radiation (case 4). The other four patients showed 'evident' radiological response and three of them died from the progression of primary lesion at 2, 4 and 15 months, respectively.
The six craniopharyngioma patients showed the most reliable response. All six patients showed the improvement of their headache or visual acuity during the follow-up period. Two patients showed decrease of both the cyst volume and the wall thickness to about half of pre-treatment one (case 19 and 20). One of the patients maintained static status until third injection when 'suggestive' response was seen at 15 months from initial therapy and 2 months after fourth injection (case 16). Two other patients revealed 'suggestive' radiological response with clinical improvement. One patient maintained clinical response up to 10 months and lost (case 17) and the other patient revealed radiological recurrence at 12 months (case 21). The other patient showed no significant radiological change but her headache and visual acuity was improved without any additional therapy for 10 months (case 18). One trigeminal schwannoma patient showed stable status but ultimate response was hard to estimate because follow-up had been lost at 11 month.
DISCUSSION
Ho-166-chico as an ideal radioisotope for intracavitary radiation
An ideal radioisotope for internal irradiation should have the properties of a short half-life, easy applicability and pure beta emitting 9). Among various isotopes used for internal irradiation, Holmium-166 has the ideal properties of a half-life of 27 hours and 94% beta emission. It has a maximum energy release of 1.85 MeV and a maximum penetrance of 8.5 mm, comparable to P-32 (1.71 MeV and 7.9 mm, each). Radioisotopes, using colloid as carrier, posed problems of leakage through membranes owing to its low molecular weight and possibility of foreign body reaction. In a pilot study, using a biodegradable Poly (L-lactic acid) microsphere as carrier of Holmium-166, this complex showed a good retention rate (94%) in the liver and stable biodegradation after the complete decay of the Holmium-166 15). But the condition for preparation of this complex was very difficult (-23℃) with low yield rate (36%), preventing wide use of this complex. Chitosan is a deacetylated polymer of chitin [poly-β(1-4)-N-acetyl-D-glucosamine], which is a naturally existing amino-polysaccharide in the shell of crab, shrimp and at the bone of cuttlefish. Commercially available chitosan (Samcheon Ri Pharm. Co.) is a 98% deacetylated form of molecular weight of 500000 Dalton. Free amino group of chitosan can be easily made to a complex with Ho-166(NO 3)3·5H 2O via chelate formation at room temperature. This biocompatible Ho-166-chitosan complex remained completely in the cystic cavity in vivo for more than 48 hours (99.7% of injected amount) and biodegraded completely in time without provoking any foreign body reaction 18). However, its bioaffinity poses some problems in calculating exact dosimetry in the cystic cavity because it preferentially bound to the cyst membrane. The dose difference according to the bound proportion could be more than 10 times as the cyst diameter becomes larger ( Fig. 2). Our calculation of bound proportion is the first data revealing stable, near complete bound (>92%) of Ho-166-chico to the cyst wall at 12 hours. However, a difference of time to be bound is suggested according to the tumor type.
Complications of intracavitary radiation for cystic brain tumors
Complications associated with intracavitary radiation have been reported mostly as cranial neuropathy in cases abutting to critical structures 7,9). Thus, the possible mechanism of complications could be assumed to be either radiation-damage thorough thin cyst wall or leakage of injected radioactive material. To prevent the leakage, many authors used pre-installed intracavitary catheter with or without leakage test. Some authors directly injected radio-active substances at the field of craniotomy or stereotactic aspiration 7,14). However, immediate complications probably due to the leakage were not neuropathies but transient constitutional symptoms such as fever, dizziness and anorexia. Julow et al. 7) described transient oculomotor paresis occurred some months after radioisotope installation. Kobayashi et al. 9) reported immediate nausea, vomiting and blurred vision followed by progression of oculomotor palsy over weeks but CSF examination revealed no evidence of leakage into CSF space. Both were recurrent craniopharyngioma and they insisted the adhesion of oculomotor nerve to thin walled cyst is responsible for radiation-injury. In our study, one case of transient oculomotor palsy (case 19) and another case of permanent partial oculomotor palsy (case 21) were recurrent craniopharyngiomas. The wall thickness of permanent paresis case was as thin as 1 mm. Thus, caution should be given when treating thin walled cyst abutting to critical neural structures and again, repeated injection of minimal dose of radioisotope via subcutaneous reservoir is important to avoid complications of intracavitary radiation 6,21).
Dosimetry for intracavitary radiation
The most important factor in internal irradiation should be the determination of the minimum effective radiation to the cyst wall of varying thickness 9). The major factors determining the radiation dose to the cyst wall at a given dose of isotope are cyst volume, the degree of isotope dispersion, and proportion of the bound form. The cyst volume was the determining factor of isotope density. Some of the authors deduced the cyst volume from the diameter of the cyst on CT or MR images 14). This volumetric method usually gave an approximated volume under assumption that the cyst was a spherical or ellipsoidal shape, but a more precise result could be achieved with a radionuclide dilution method 6,12). Under the assumption of uniform dispersion of the colloid, the cyst volume influences the radiation dose in a just proportional scale ( Fig. 2). However, when using the isotope with a macromolecular carrier, a considerable portion of a given isotope was bound to the cyst wall. Even in the case of colloidal isotope, re-aspiration of the cyst after 14 to 70 days of the injection disclosed that considerable portion of isotope was adhered to the cyst wall 19). The proportion of the bound form can be calculated from the remaining activity of the isotope in cystic fluid. In our study, approximately 98% of the remaining isotope was bound to the cyst wall at 12 hours. This result suggests that without consideration of bound portion of initially dispersed isotope, the actual radioactivity can be underestimated. Thus, a sequential sampling for measuring the actual radioactivity of the bound form is necessary to calculate the exact dose distribution of a given isotope. The optimum dose of internal irradiation of cystic tumor was to alleviate fluid retention or to cause collapse of a cyst with minimal damage to adjacent structure 7,9). Kobayashi et al. 9) did a fairly reasonable observation of the effect of cyst wall thickness for dose distribution by including it as one of parameters in their dosimetric formula. In our series, we used not a dose to the cyst wall, but a dose to a depth of 4 mm beyond the inner cyst wall as a target dose to reduce potential damage to adjacent vital structures.
Measurement of the response and clinical implication
The cystic brain tumors have been considered a non-measurable lesion for treatment response due to lack of agreed parameters 26). Some investigators recognized problems of cystic lesions including pseudo-progression and tried to include the tumor wall thickness as a parameter 5). However, the wall thickness is usually too thin (<10 mm) to give a significant difference. Kim et al. 8) tried to evaluate the efficacy of Ho-166-chico for renal cyst by measuring the decrease of cyst volume but renal cyst does not contain tumorous wall. As in our series, the cyst aspiration was performed in random interval according to clinical need. Thus, the cyst volume alone could not be considered to be a response as the volume of the solid tumor. Many authors considered symptomatic improvement, recurrence free-survival, reduced rate of cystic fluid accumulation or the cyst size as a response of the treatment 1,9,23). However, these parameters are hard to be considered the direct effect of the radiation to the tumor cyst. As far as we reviewed in the literature, many studies, based on recurrence free survival or reduced rate of mandatory cystic fluid aspiration, were neither performed by prospective protocol nor had a comparable control group. Our study is the first attempt to estimate the direct effect of the treatment by measuring the change of cyst wall thickness after the treatment. Though it was not easy to measure the change of thickness in thin walled cyst, half of our treated cases showed measurable changes. The wall thickness change was a more reliable variable to reflect the activity of lining tumor cells and a total tumor burden. In our series, these changes could be seen at as early as one-month follow-up images in malignant tumor. As radiation response appeared earlier in malignant tumors than benign slow growing tumors, malignant tumors such as lung cancer and/or breast cancer showed radiological response as we evaluated one month after the treatment. However, most of these initially responded malignant tumors recurred later, and repeated injection with fixed intervals is necessary to prevent progression. Taasan et al. 25) insisted that the single isotope instillation is too small dose or too short of biological effect to control malignant glioma, although it apparently has some clinical benefit. With benign tumors like craniopharyngioma, a single isotope installation has some clinical effect on the activity of lining tumor epithelium. Considering conventional radiation therapy for craniopharyngioma always carries the risk of pituitary insufficiency in long-term result, the effectiveness of intracavitary radiation for benign cystic tumor, especially for cystic craniopharyngioma, have been verified by many authors 1,6,7,9,10,12,14,19,22,23). In our series, all benign cystic tumors including low grade glioma showed clinical response and some reluctant craniopharyngioma revealed radiological response upon repeated injection.
CONCLUSION
The intracavitary radiation using Ho-166-chico for recurrent cystic brain tumors showed 70% of radiological response and prolonged tumor control in both benign tumors and low grade astrocytomas with clinical improvement. Measurement of radioactivity of the cystic fluid revealed that most of (>92%) Ho-166-chico bound to the cyst wall at 12 hours and systemic absorption in 24 hours urine and blood sample was negligible.
Although a more precise estimation of radiation delivery is needed, a repeated intracavitary radiation of low dose Ho-166-chico could be considered one of useful methods for treating clinically difficult cystic brain tumors.
References
1. Backlund EO, Johansson L, Sarby B : Studies on craniopharyngiomas. II. Treatment by stereotaxis and radiosurgery. Acta Chir Scand 1972, 138 : 749-759,  2. Burton MA, Gray BN, Klemp PF, Kelleher DK, Hardy N : Selective internal radiation therapy : distribution of radiation in the liver. Eur J Cancer Clin Oncol 1989, 25 : 1487-1491,   3. Fox JL : Intermittent drainage of intracranial cyst via the subcutaneous Ommaya reservoir. Technical note. J Neurosurg 1967, 27 : 272-273,   4. Gutin PH, Klemme WM, Lagger RL, MacKay AR, Pitts LH, Hosobuchi Y : Management of the unresectable cystic craniopharyngioma by aspiration through an Ommaya reservoir drainage system. J Neurosurg 1980, 52 : 36-40,   5. Henson JW, Ulmer S, Harris GJ : Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol 2008, 29 : 419-424,    6. Huk WJ, Mahlstedt J : Intracystic radiotherapy (90Y) of craniopharyngiomas : CT-guided stereotaxic implantation of indwelling drainage system. AJNR Am J Neuroradiol 1983, 4 : 803-806,    7. Julow J, Lányi F, Hajda M, Simkovics M, Arany I, Tóth S, et al : The radiotherapy of cystic craniopharyngioma with intracystic installation of 90Y silicate colloid. Acta Neurochir (Wien) 1985, 74 : 94-99,   8. Kim JH, Lee JT, Kim EK, Won JY, Kim MJ, Lee JD, et al : Percutaneous sclerotherapy of renal cysts with a beta-emitting radionuclide, holmium-166-chitosan complex. Korean J Radiol 2004, 5 : 128-133,    9. Kobayashi T, Kageyama N, Ohara K : Internal irradiation for cystic craniopharyngioma. J Neurosurg 1981, 55 : 896-903,   10. Lange M, Kirsch CM, Steude U, Oeckler R : Intracavitary treatment of intrasellar cystic craniopharyngeomas with 90-Yttrium by trans-sphenoidal approach--a technical note. Acta Neurochir (Wien) 1995, 135 : 206-209,   11. Lee JD, Park KK, Lee MG, Kim EH, Rhim KJ, Lee JT, et al : Radionuclide therapy of skin cancers and Bowen's disease using a specially designed skin patch. J Nucl Med 1997, 38 : 697-702,  12. Leksell L, Backlund EO, Johansson L : Treatment of craniopharyngiomas. Acta Chir Scand 1967, 133 : 345-350,  13. Leksell L, Liden K : A therapeutic trial with radioactive isotopes in cystic brain tumor. Atomic Energy Research EstablishmentRadioisotope Techniques : Medical and physiological applications. London : H.M. Stationery Office, 1951, Vol 1.
14. Liu Z, Tian Z, Yu X, Li S, Huang H, Kang G, et al : Stereotactic intratumour irradiation with nuclide for craniopharyngiomas. Chin Med J (Engl) 1996, 109 : 219-222,  15. Mumper RJ, Ryo UY, Jay M : Neutron-activated holmium-166-poly (L-lactic acid) microspheres : a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med 1991, 32 : 2139-2143,  16. Muzzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G, Gazzanelli G, et al : Biological activity of chitosan : ultrastructural study. Biomaterials 1988, 9 : 247-252,   17. Nijsen JF, Zonnenberg BA, Woittiez JR, Rook DW, Swildens-van Woudenberg IA, van Rijk PP, et al : Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies : effects of preparation and neutron activation techniques. Eur J Nucl Med 1999, 26 : 699-704,   18. Park KB, Kim YM, Shin BC, Kim JR : Study on the preparations of new 166Ho-chitosan complex and its macroaggregates for a potential use of internal radiotherapy. Korean J Nucl Med 1996, 30 : 351-360,
19. Pollack IF, Lunsford LD, Slamovits TL, Gumerman LW, Levine G, Robinson AG : Stereotaxic intracavitary irradiation for cystic craniopharyngiomas. J Neurosurg 1988, 68 : 227-233,   20. Prestwich WV, Nunes J, Kwok CS : Beta dose point kernels for radionuclides of potential use in radioimmunotherapy. J Nucl Med 1989, 30 : 1036-1046,  21. Rogers LR, Barnett G : Percutaneous aspiration of brain tumor cysts via the Ommaya reservoir system. Neurology 1991, 41( Pt 1):279-282,   22. Strauss L, Sturm V, Georgi P, Schlegel W, Ostertag H, Clorius JH, et al : Radioisotope therapy of cystic craniopharyngomas. Int J Radiat Oncol Biol Phys 1982, 8 : 1581-1585,   23. Sturm V RT, Strauss L : Preliminary results of intracavitary irradiation of cystic craniopharyngiomas by means of stereotactically applied yittrium-90. Edited by Schiefer W KM, Brock M : In: Advances in Neurosurgery. Berlin : Springer-Verlag, 1981, Vol 9 : pp401-404
24. Suzuki YS, Momose Y, Higashi N, Shigematsu A, Park KB, Kim YM, et al : Biodistribution and kinetics of holmium-166-chitosan complex (DW-166HC) in rats and mice. J Nucl Med 1998, 39 : 2161-2166,  25. Taasan V, Shapiro B, Taren JA, Beierwaltes WH, McKeever P, Wahl RL, et al : Phosphorus-32 therapy of cystic Grade IV astrocytomas : technique and preliminary application. J Nucl Med 1985, 26 : 1335-1338,  26. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al : Updated response assessment criteria for high-grade gliomas : response assessment in neuro-oncology working group. J Clin Oncol 2010, 28 : 1963-1972,  
Fig. 1
Leakage test before Holmium-166-chitosan inject. Omnipaque® contrast agent is directly injected to the tumor cyst and CT scan reveals the exact tumor volume and any possible leakage. 
Fig. 2
Calculated absorbed dose (Gy) to a 1 mm-thick cyst wall at a varying depth for each diameter cyst by Monte-Carlos simulation, when 10 mCi/mL of Holmium-166-chitosan uniformly distributed in the cystic fluid (empty bullet) and/or uniformly bound to the cyst wall surface (filled bullet). 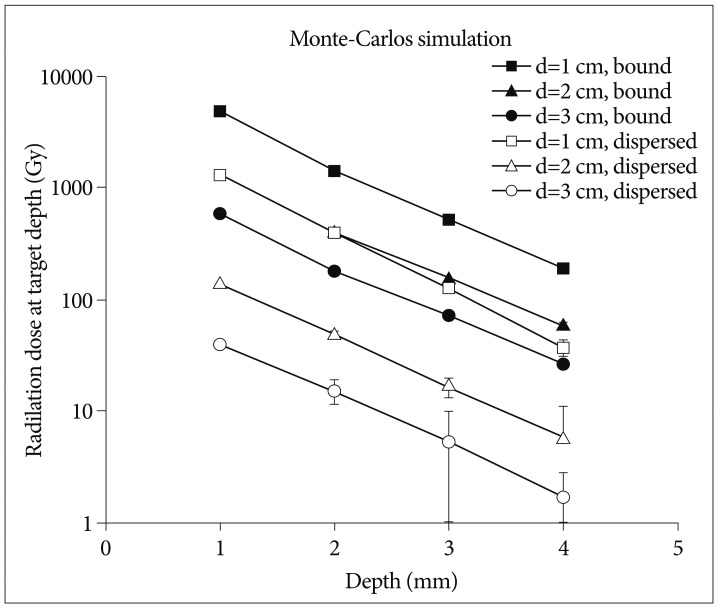
Fig. 3
The distribution of injected Holmium-166-chitosan to the tumor cyst is confirmed by gamma camera. 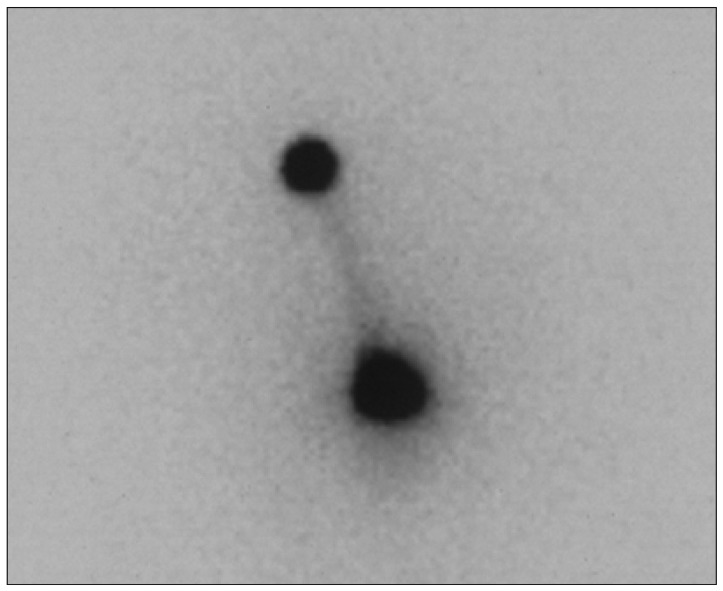
Fig. 4
The proportion of wall bound form is depicted by time after Holmium-166-chitosan injection. 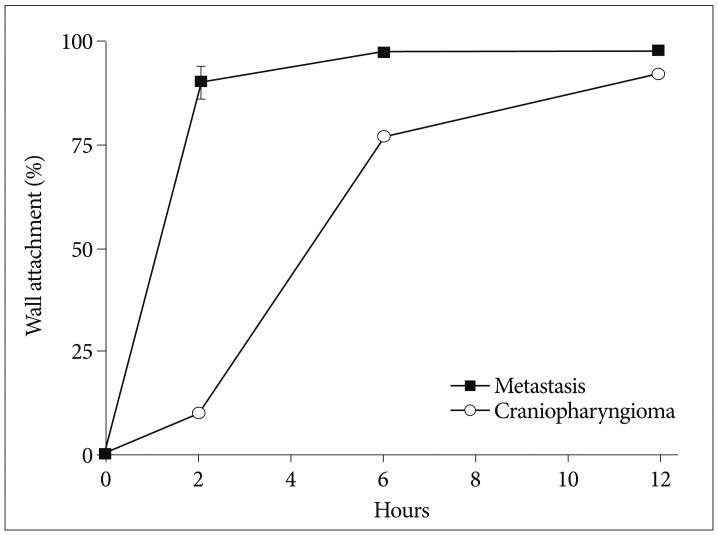
Fig. 5
Illustrative case of 'evident' radiological response. A : MRI before the treatment reveals cystic brain metastasis at motor cortex. The patients suffered from transient hemiparesis at 1st Holmium-166-chitosan (Ho-166-chico) injection of 15 mCi. However, he recovered completely with steroid treatment and underwent two more Ho-166-chico therapy. B : MRI follow-up 11 months later showed significant decrease of both cyst volume and wall thickness. 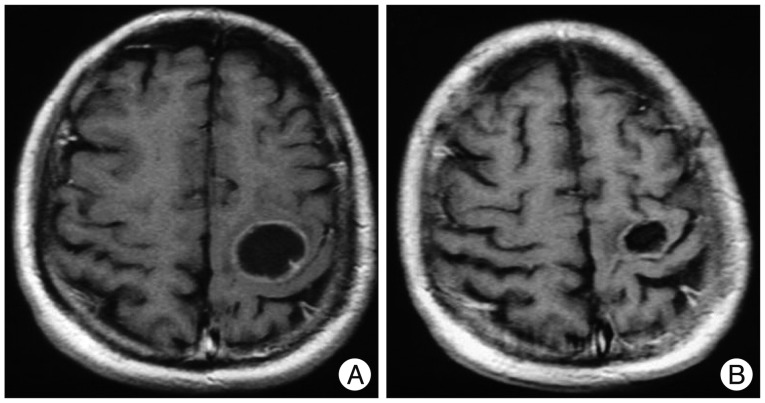
Fig. 6
Another illustrative case of 'evident' radiological response. A : Sagittal enhanced MRI before the treatment reveals enhancing cystic craniopharyngioma with mural nodule anteriorly. B : After 4 mCi of Holmium-166-chitosan intracavitary injection, MRI follow-up 3 months later showed definite decrease of the cyst volume and the mural nodule. Later, the cyst recurred at 36 months after the treatment. 
Table 1
Treatment parameters with radiological response 
Table 2
Clinical characteristics with follow-up result 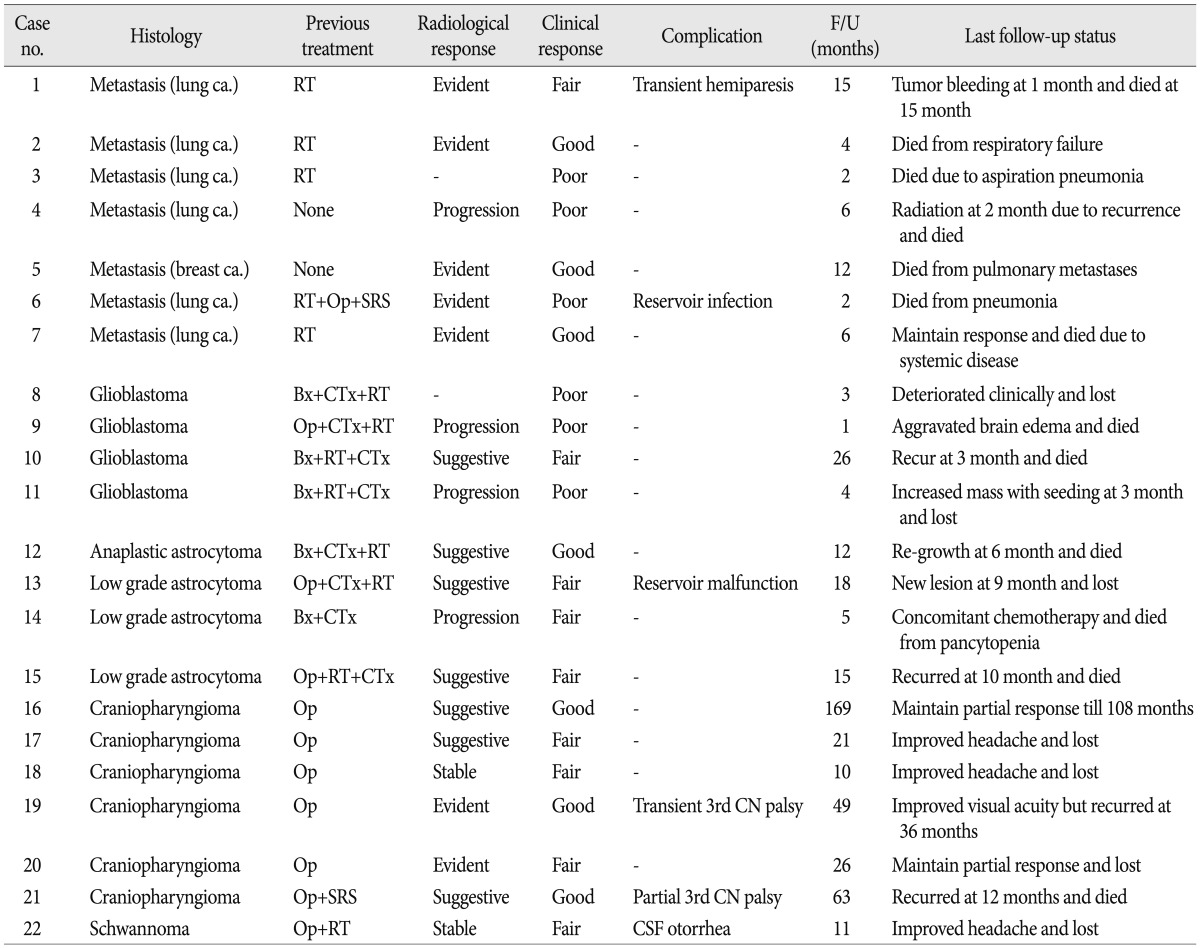
|
|























