Evaluation of Non-Watertight Dural Reconstruction with Collagen Matrix Onlay Graft in Posterior Fossa Surgery
Article information
Abstract
Objective
Many surgeons advocate for watertight dural reconstruction after posterior fossa surgery given the significant risk of cerebrospinal fluid (CSF) leak. Little evidence exists for posterior fossa dural reconstruction utilizing monolayer collagen matrix onlay graft in a non-watertight fashion. Our objective was to report the results of using collagen matrix in a non-watertight fashion for posterior fossa dural reconstruction.
Methods
We conducted a retrospective review of operations performed by the senior author from 2004–2011 identified collagen matrix (DuraGen) use in 84 posterior fossa operations. Wound complications such as CSF leak, infection, pseudomeningocele, and aseptic meningitis were noted. Fisher's exact test was performed to assess risk factor association with specific complications.
Results
Incisional CSF leak rate was 8.3% and non-incisional CSF leak rate was 3.6%. Incidence of aseptic meningitis was 7.1% and all cases resolved with steroids alone. Incidence of palpable and symptomatic pseudomeningocele in follow-up was 10.7% and 3.6% respectively. Postoperative infection rate was 4.8%. Previous surgery was associated with pseudomeningocele development (p<0.05).
Conclusion
When primary dural closure after posterior fossa surgery is undesirable or not feasible, non-watertight dural reconstruction with collagen matrix resulted in incisional CSF leak in 8.3%. Incidence of pseudomeningocele, aseptic meningitis, and wound infection were within acceptable range. Data from this study may be used to compare alternative methods of dural reconstruction in posterior fossa surgery.
INTRODUCTION
The incidence of cerebrospinal fluid (CSF) leak after posterior fossa surgery ranges between 1–14% in the literature456815212527). Factors associated with an increased risk of CSF leak in posterior fossa cases include larger tumor size425), post-operative meningitis26), re-operation29), and craniectomy rather than craniotomy8). CSF leak has also been associated with significant medical costs due to prolonged hospital stay and need for additional interventions9). Pseudomeningoceles without CSF leak can present with cosmetic deformity and debilitating symptoms such as positional headache. The incidence of clinically relevant pseudomeningocele in the literature ranges from 4–23% 515212527).
Posterior fossa surgery is associated with a higher incidence of CSF leak than supratentorial surgery likely because of large adjacent CSF cisterns, strain on dural closure in recumbency, and increased rate of post-operative hydrocephalus compared to supratentorial surgery. Classically, watertight dural closure has been advocated to prevent hydrodynamic complications. However, even when watertight primary dural closure is attempted, it is frequently not achieved3). In supratentorial surgery, one randomized trial found no benefit of watertight dural closure over non-watertight adaptive closure consisting of interrupted simple sutures placed 2–3 cm apart1). However, results from supratentorial cases may not apply to the posterior fossa for the aforementioned reasons.
In many cases, primary dural closure is not feasible due to inadvertent dural damage, shrinkage during prolonged surgery, intended resection in tumor invasion, or intended expansion duraplasty for posterior fossa decompression. Many types of dural substitutes have been used for reconstruction including synthetic materials142332), synthetic collagen matrix 13171819), dense collagen autografts 111230), xenografts27), and allografts533). In addition to dural grafts, multiple sealants have been utilized as adjuncts to obtaining watertight dural closure2022). Still, the optimal dural reconstruction strategy for minimizing complications in posterior fossa surgery has not been defined.
DuraGen (Integra Lifesciences, Plainsboro, NJ, USA) is a sutureless dural substitute graft composed of purified type I collagen extracted from bovine Achilles tendon. The collagen matrix provides a scaffold for invasion of host fibroblasts, promotes fibrin clot, and is fully reabsorbed as the wound heals. The purpose of this study was to determine the incidence of hydrodynamic complications for posterior fossa dural reconstruction, when primary dural closure is undesirable or not feasible, using collagen matrix in a non-watertight fashion.
MATERIALS AND METHODS
Patient population
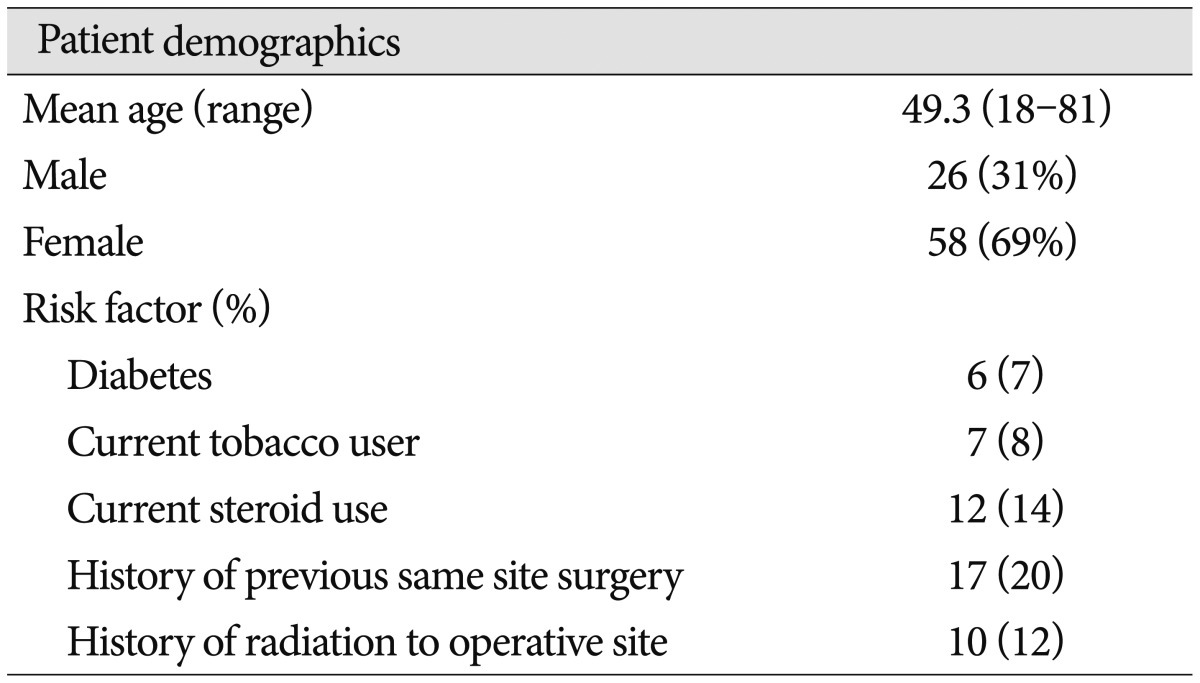
A retrospective chart review was completed on patients who underwent posterior fossa surgery by the senior author at the Cleveland Clinic from January 2004 to May 2011. Eighty-four posterior fossa operations utilizing collagen matrix were identified. Collagen matrix was used when dural defects were noted after dural approximation or purposeful dural resection had been completed. Patient demographics, preoperative risk factors, lesion pathology, and operative approach were recorded. The Cleveland Clinic Institutional Review Board approved this study.
Surgical technique
After the posterior fossa operation has been carried out, the dural edges are approximated. If a dural defect was present, an appropriately size collagen matrix graft was selected to cover the defect and at least 5 millimeters of native dura. In cases of very large dural defects, a few interrupted simple sutures are used to bridge the native dura and prevent the graft from falling through the dural defect. Although meticulous intradural hemostasis is always completed, a minimal amount of self-limiting extradural bleeding is preferred since it provides fibroblasts for the infiltration of the collagen matrix. The margins of the collagen matrix graft are reinforced with stamp size pieces of absorbable compressed gelatin sponge (Gelfoam®, Pharmacia & Upjohn Company, Kalamazoo, MI, USA) soaked in the patient's blood. These provide fibrin that secures the graft in place without the need for any exogenous sealants. We do not use sealants as these can theoretically prevent migration of fibroblasts into the dural graft. After the graft has been appropriately placed and secured, either cranioplasty or replacement of bone flap is performed. No drains were used.
Complication assessment and management
Charts were reviewed for wound complications for up to 24 months after surgery. Recorded post-operative wound complications included CSF leak, clinically-evident pseudomeningocele, surgical site infection, aseptic meningitis, and need for permanent CSF diversion.
CSF leak was defined as clear or blood tinged fluid egression outside the wound and subdivided into incisional CSF leak or non-incisional CSF leak (otorrhea or rhinorrhea). Pseudomeningocele was defined as an extra-axial fluid collection causing cosmetic deformity noted on follow-up postoperative physical examination. Low attenuation extra-axial fluid collections noted on immediate postoperative imaging are common using our technique since a watertight closure is not pursued. Therefore, we excluded any solely radiographic pseudomeningoceles since these are small, asymptomatic, and do not require any intervention.
Wound infection was noted in any patient who was treated with antibiotics aimed to treat wound breakdown, purulent drainage, or systemic signs presumed to be secondary to surgical site infection regardless of whether or not any clinical pathogen was isolated. Patients who were treated with intravenous antibiotics and had signs of meningismus or an isolated CSF microbe were classified as a deep surgical site infection. All others were grouped into superficial site infections and treated with oral antibiotics.
Diagnosis of aseptic meningitis was restricted to patients that had meningismus and headaches that improved with steroids, or CSF leukocytosis without positive cultures for patients not on antibiotics. Aseptic meningitis was treated with a prolonged course of oral steroids.
Wound complications were categorized as those primarily related to collagen matrix use (incisional CSF leak, pseudomeningocele formation, and aseptic meningitis) and those that unrelated to collagen matrix use (superficial wound infection, meningitis, and CSF rhinorrhea). Although aseptic meningitis can occur after posterior fossa surgery regardless of collagen matrix use, we categorized this complication as attributable to the collagen matrix because it was not readily differentiated from an inflammatory reaction against a foreign body. CSF rhinorrhea was categorized as a complication primarily attributable to surgery since it represents inadequate repair of the mastoid air cells at the time of initial surgery.
Statistical analysis
A one-sided Fisher's exact test was used to determine if there was any significant association between risk factors and wound complications. Significance level was <0.05.
RESULTS
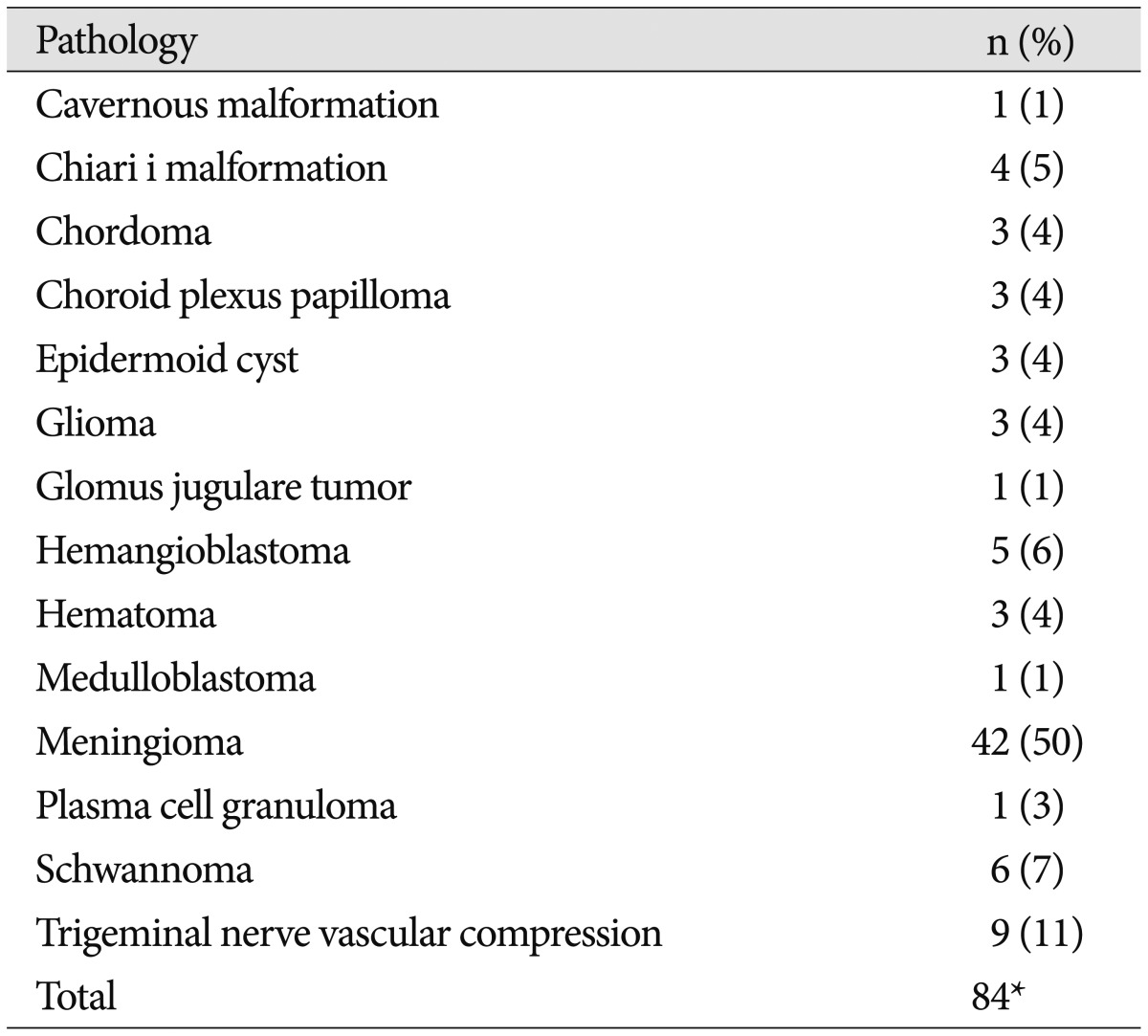
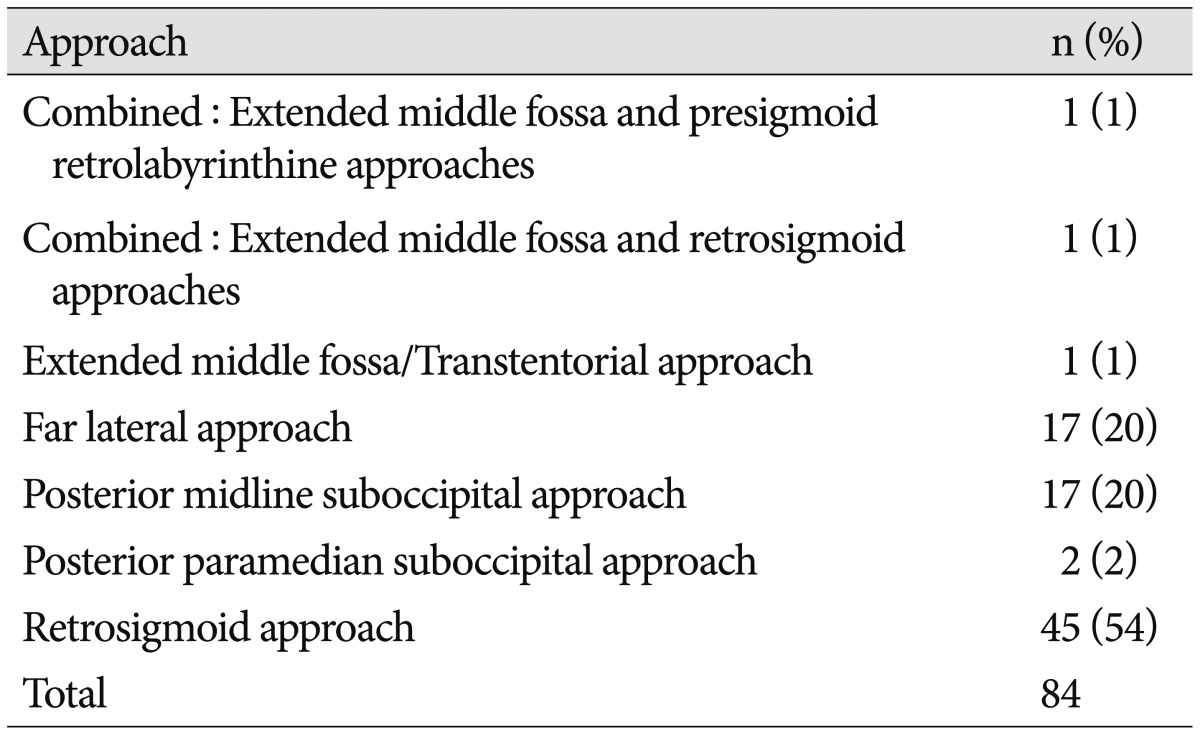
Collagen matrix was used in 84 posterior fossa operations in 78 patients. Case demographics and risk factors are listed in Table 1. Ages ranged from 18–81 years old (mean 49.4 years). The majority (69%) of cases were on female patients. The most common operative pathology was meningioma (50%), followed by trigeminal nerve vascular compression (11%), and schwannoma (7%) (Table 2). The most common operative approaches were retrosigmoid (54%), far lateral (20%), and posterior midline suboccipital (20%) (Table 3). Average duration of follow-up was 14 months (range 1–24 months) with 31 (40%) cases followed to 24 months after surgery.
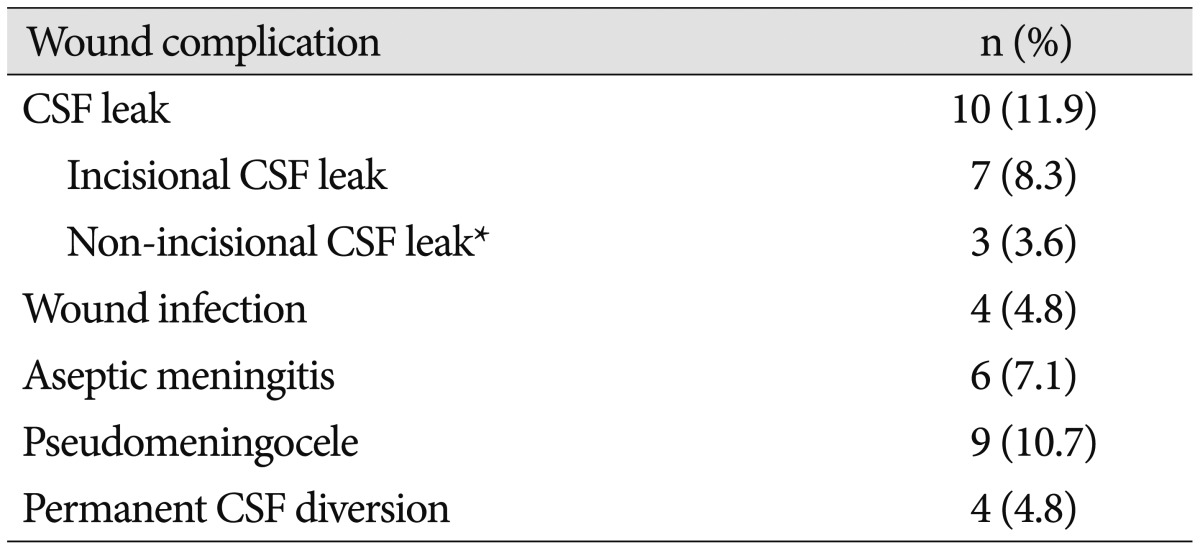
Complications are listed in Table 4. Wound complications related to collagen matrix use were noted after 14 cases (16.6%). Seven cases (8.3%) had complications that were unrelated to collagen matrix use. The mean time until a complication was noted was 49 days (range 5–362 days) with 75% of complications occurring within 50 days after surgery.
Wound complications related to collagen matrix
There were 7 (8.3%) incisional CSF leaks. Three resolved simply with oversewing, two required temporary CSF diversion with a subarachnoid drain, and one ultimately required permanent CSF diversion to prevent CSF wound leakage. The patient that required permanent CSF diversion had preoperative obstructive hydrocephalus secondary to her fourth ventricle glioma and had persistent hydrocephalus despite resection of her tumor. No patient with an incisional CSF leak had an associated infection.
Six (7.1%) cases were treated for suspected aseptic meningitis. These were all treated with a course of steroids. No patient was treated with antibiotics. Of note, one of the patients with aseptic meningitis without CSF leak required 6 months of oral steroids to control postoperative pressure-type headaches in the setting of CSF leukocytosis with negative cultures and negative imaging for hydrocephalus.
Clinically-evident pseudomeningoceles were noted after nine operations (10.7%). Only three of these patients (3.6% of total cases) were symptomatic with headaches. Of the nine patients with pseudomeningoceles, one was lost to follow-up, one required permanent CSF diversion for delayed onset communicating hydrocephalus and the other seven had complete resolution without intervention.
Overall, out of the 14 cases with complications attributed to collagen matrix use, ten patients had complete resolution of their complications when treated conservatively with observation, medical management, and/or simple outpatient procedure (suturing of wound), and four required a more invasive procedure (temporary and/or permanent CSF diversion).
Wound complications not related to collagen matrix use
Non-incisional CSF leaks occurred in 3 (3.6%) cases, all of which manifested as rhinorrhea and were believed to secondary to inadequate surgical repair of the mastoid air cells at the time of initial surgery. Of these patients, two resolved with observation alone, and one patient required surgical repair. Four (4.8%) postoperative wound infections were noted, of which three were superficial infections, and one was meningitis.
Risk factor analysis
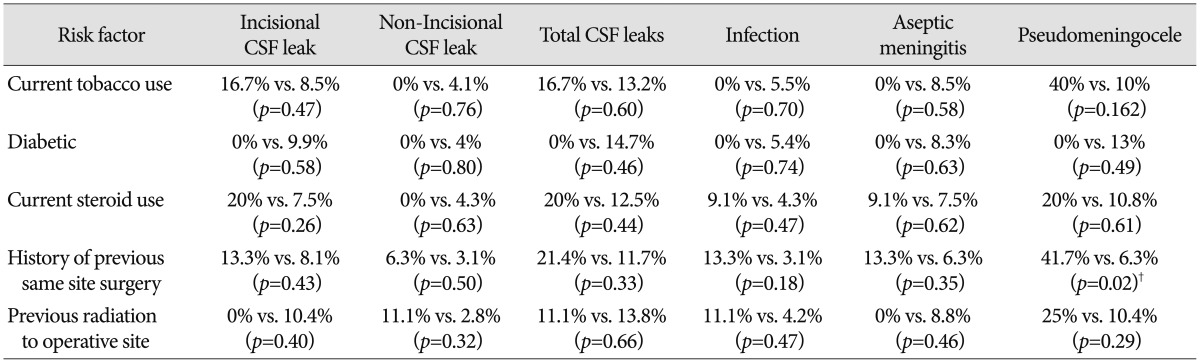
The association of complications with preoperative risk factors is listed in Table 5. Prior surgery was significantly associated with an increased rate of postoperative pseudomeningocele (41.7% vs. 6.3%, p<0.05). No other statistically significant associations were found. To further evaluate the relationship between surgical approach and incisional CSF leak, we divided surgical approaches into those adjacent and not adjacent to a fourth ventricular outflow tract. The former included the midline suboccipital (adjacent to the foramen of Magendie) and the far lateral approach (adjacent to the foramen of Luschka). The incisional CSF leak rate was 14.7% in those approaches adjacent to a fourth ventricular outflow tract compared to 4.0% in all other posterior fossa approaches (p=0.091).
DISCUSSION
Much effort has been given to reducing the incidence of CSF leak following posterior fossa surgery. There is some suggestion in the literature that autologous grafts may have the lowest rate of hydrodynamic complications. Kosnik reported on the technique for nuchal ligament harvest for posterior fossa duraplasty and informally reported no CSF leak or pseudomeningocele in 200 cases11). In a large series of 500 pediatric Chiari decompressions, autologous pericranium was used in 91% cases, with only a single case of CSF leak31). Lam and Kasper12) recently reported on 100 posterior fossa cases of various pathology where they achieved a 1% CSF leak rate using a combination of pericranial autograft, Surgicel™, polyethylene glycol (PEG) hydrogel sealant, and thrombin soaked Surgifoam™.
However, the drawbacks of autologous grafts include donor site morbidity, and in cases of repeat surgery, paucity of appropriate local tissue. For this reason, various dural substitutes have been investigated. The ideal substitute is one that is easy to prepare and handle, allows enough durability while maintaining flexibility, and does not adhere to the brain nor incite any host inflammation or neurotoxicity. Collagen sponges have thus far provided a promising option for a dural substitute and have been extensively studied. These sponges have a large surface area for CSF absorption, which helps the graft adhere to dura via surface tension24). Blood on the sponge allows deposition of fibrin, which holds the graft in place in the immediate time period1824). Fibroblasts begin to proliferate around 3–4 days and are established by 10–14 days. Over the next few weeks, fibroblasts deposit collagen onto the matrix pores and neovascularization of the graft continues18). By 6–8 weeks, the collagen matrix is reabsorbed18). Of note, Narotam et al.18) performed a histologic evaluation of implanted collagen sponges in over 100 reoperations and autopsies and did not find any evidence of inflammatory cell infiltration, reactive encapsulation, or inflammatory reaction in the underlying brain sections.
Previous reports have demonstrated that collagen matrix provides a safe and effective method of dural reconstruction in supratentorial and spinal surgery 101316182428). Specific reports of collagen matrix use after posterior fossa surgery are few. Litvack et al.13) used monolayer or bilayer (suturable) collagen matrix with or without sealants in 150 infratentorial cases. They had an 11.3% rate of either CSF leak or clinically relevant pseudomeningocele and found a statistically higher risk of CSF leak or clinically relevant pseudomeningocele when monolayer collagen matrix was used instead of bilayer collagen matrix. However, the specific CSF leak rate for monolayer collagen matrix and the frequency of use of monolayer collagen matrix versus bilayer collagen within the posterior fossa surgery subgroup was not reported. Conversely, Moskowitz et al.15) reported a 25% CSF leak and 14.2% pseudomeningocele rate in 28 patients with bilayered collagen matrix compared to 10% CSF leak and 4.2% pseudomeningocele rate in 70 patients with monolayer collagen matrix.
Danish et al.5) reported a series of 101 patients undergoing Chiari decompression. Fifty-six patients had monolayer collagen matrix onlay duraplasty resulting in 1.8% CSF leak and 8.9% clinically evident pseudomeningocele. This was compared to 45 patients who had sutured duraplasty using acellular human dermis allograft resulting in 2.2% CSF leak and 11.1% pseudomeningocele incidence. The only statistically significant difference between the groups was an average of 37 minutes shorter operating time for the collagen matrix. Narotam et al.18)presented an initial series in 1995 obtaining a 4.4% CSF leak rate using a collagen sponge in 67 posterior fossa cases. In 2009, Narotam et al.17) presented a series of 52 posterior fossa operations using DuraGen, demonstrating no cases of CSF leak and pseudomeningocele in 3.8% of cases. Of note, in this series a subgaleal closed suction drain was used in 44% of cases for 48 hours postoperatively17). The discrepancy in the rate of hydrodynamic complications between this series and ours may be related to a difference in the presence of preoperative risk factors such as prior surgery and/or radiation. In addition, ventriculostomy catheters were used perioperatively in 31% of cases in the Narotam et al. study. Finally, craniectomy was used in our study compared to craniotomy in the Narotam et al study. Craniotomy in the posterior fossa has been associated with a decreased risk of postoperative CSF leak in prior studies8).
Overall, in the literature, the use of monolayer collagen matrix appears to have similar rates of hydrodynamic complications to watertight methods of dural closure 682127). However, based on the results of this study, it appears that dural reconstruction with collagen onlay graft has a higher rate of incisional CSF leak in surgical approaches adjacent to a fourth ventricular outflow tract (i.e., midline suboccipital and far lateral approaches). Prior published studies evaluating various dural reconstruction techniques after posterior fossa surgery generally have not reported CSF leak rates outcomes according to surgical approach.
Some studies have evaluated the use of dural sealants in conjunction with collagen matrix in the posterior fossa. Litvack et al.13) found a significantly increased risk of CSF leak when collagen matrix was used with fibrin glue (10.8%) or PEG hydrogel (13.2%) compared to alone (4.4%). Such sealants can theoretically impede the influx of fibroblasts in the collagen matrix. The utilization of blood-soaked Gelfoam at the edges of the collagen matrix graft may provide a simple and cost-effective alternative to expensive sealants for graft adherence.
Limitations
There were several important limitations to our study. This study represents the results of a single surgeon from a single institution. In addition, we compare our surgical results to historical controls, thus limiting the ability to draw definitive conclusions. In particular, our population included patients on chronic steroids, diabetes, and prior surgery or radiation to the operative site. This makes comparison to CSF leak rate in a younger and healthier population (e.g., Chiari I) difficult. Nonetheless, our data represent the largest published case series in which monolayer collagen was applied in a uniform technique in the posterior fossa. Further study is necessary in order to identify the optimal method of dural reconstruction after posterior fossa surgery.
CONCLUSION
Monolayer collagen matrix applied in an onlay fashion is a simple method for dural reconstruction when primary repair is undesirable or not feasible following posterior fossa surgery. This method produced an 8.3% incidence of incisional CSF leak. Additional complications such as pseudomeningocele, aseptic meningitis, and wound infection were within acceptable range. Further study is needed in order to determine the optimal strategy for dural reconstruction in the posterior fossa.