Clinical Outcomes of Gamma Knife Radiosurgery for Metastatic Brain Tumors from Gynecologic Cancer : Prognostic Factors in Local Treatment Failure and Survival
Article information
Abstract
Objective
Brain metastases in gynecologic cancer (ovarian, endometrial, and cervical cancer) patients are rare, and the efficacy of Gamma Knife Radiosurgery (GKRS) to treat these had not been evaluated. We assessed the efficacy of GKRS and prognostic factors for tumor control and survival in brain metastasis from gynecologic cancers.
Methods
This retrospective study was approved by the institutional review board. From May 1995 to October 2012, 26 women (mean age 51.3 years, range 27–70 years) with metastatic brain tumors from gynecologic cancer were treated with GKRS. We reviewed their outcomes, radiological responses, and clinical status.
Results
In total 24 patients (59 lesions) were available for follow-up imaging. The median follow-up time was 9 months. The mean treated tumor volume at the time of GKRS was 8185 mm3 (range 10–19500 mm3), and the median dose delivered to the tumor margin was 25 Gy (range, 10–30 Gy). A local tumor control rate was 89.8% (53 of 59 tumors). The median overall survival was 9.5 months after GKRS (range, 1–102 months). Age-associated multivariate analysis indicated that the Karnofsky performance status (KPS), the recursive partitioning analysis (RPA) classification, and the number of treated lesions were significant prognostic factors for overall survival (HR=0.162, p=0.008, HR=0.107, p=0.038, and HR=2.897, p=0.045, respectively).
Conclusion
GKRS is safe and effective for the management of brain metastasis from gynecologic cancers. The clinical status of the patient is important in determining the overall survival time.
INTRODUCTION
Brain metastases occur in 20–40% of patients with systemic cancer, but very rarely from gynecologic cancer8293031). The incidences of brain metastasis from ovarian, endometrial, and cervical cancer have been reported to be 0.3–2.2%, 0.4–1.2%, and 0.3–0.9%, respectively25). However, the occurrence of brain metastasis from gynecologic cancer appears to have increased in recent years14172325). The cause of this increase might be due to lengthening of survival times and early diagnosis11427). Therefore, effective and appropriate treatment is essential for these patients.
It is well known that Gamma Knife Radiosurgery (GKRS) is effective to treat metastatic brain tumors36). And for brain metastasis from gynecologic cancers, many different treatments have been used, including surgery, whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS) including GKRS, and chemotherapy. Proper multimodal therapy has improved survival in patients with brain metastasis from gynecologic cancers6182325). However, in the majority of patients, treatment of brain metastasis from gynecologic cancers is palliative because the primary disease is advanced and the general condition of these patients is poor23). Therefore, SRS has recently emerged as the preferred treatment option for these types of metastasis162134).
MATERIALS AND METHODS
Patients
This retrospective study was approved by the institutional review board, and the requirement to obtain written informed consent was waived. From May 1995 to October 2012, 26 patients with metastatic brain tumors from gynecologic cancers underwent GKRS. Gynecologic cancers include ovarian, endometrial, and cervical cancer, and these diagnoses were confirmed in our patients by pathologic examination of specimens from a biopsy or surgical resection. The diagnosis of brain metastasis was confirmed by magnetic resonance imaging (MRI).
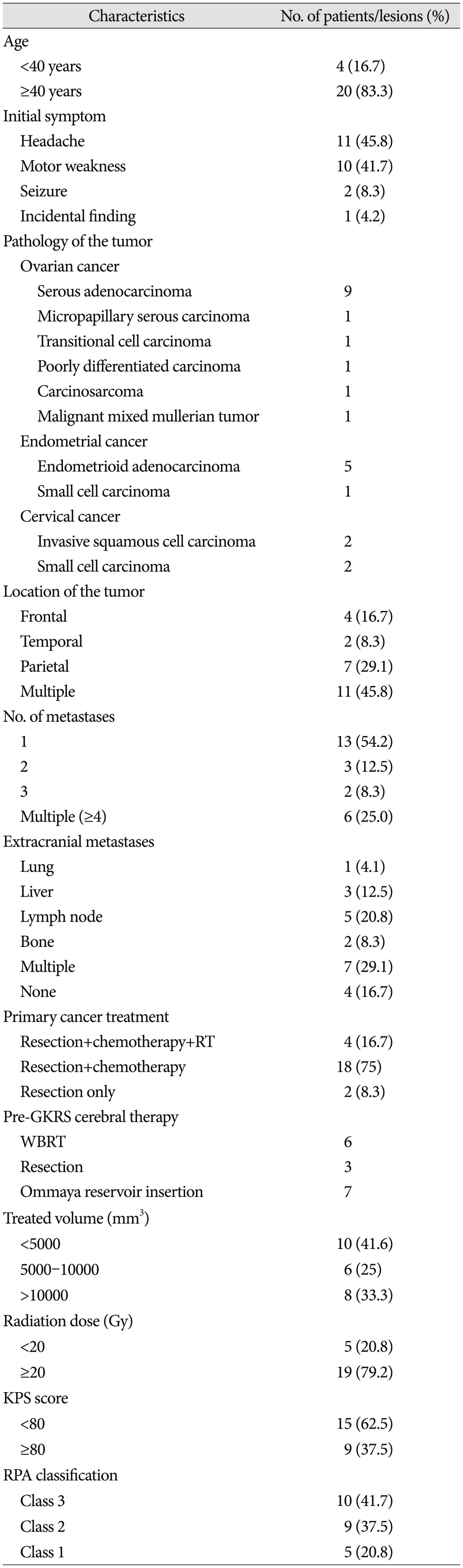
Our series of 26 patients treated by GKRS comprised 67 lesions. However, two patients were not available for follow-up imaging. The mean age of the patients was 51.3 years (range 27–70 years). Primary cancers included ovarian cancer (14 patients, 58.3%), endometrial cancer (6 patients, 25.0%), and cervical cancer (4 patients, 16.7%). Initial symptoms of metastasis were a headache (11 patients, 45.8%), motor weakness (10 patients, 41.7%), seizure (2 patients, 8.3%), and an incidental finding (1 patients, 4.2%). The mean number of metastases was 2.46 (range 1–8). All patients were classified according to the recursive partitioning analysis (RPA) classification system of the Radiation Therapy Oncology Group (RTOG)37). The mean Karnofsky performance status (KPS) score was 68.1 (range 50–90), and the RPA classification of the RTOG categorized 5 patients (20.8%) as RPA Class 1, 9 patients (37.5%) as RPA Class 2, and 10 patients (41.7%) as RPA Class 3. The mean interval from the primary site diagnosis to the brain metastasis was 42 months (range 1–168 months). The patient characteristics are summarized in Table 1.
GKRS methodology and follow-up protocol
For the GKRS, we used Leksell Gamma-Plan software. After positioning of a Leksell stereotactic frame, an MRI was performed to localize the tumor. T1-weighted MR images with gadolinium contrast enhancement were obtained from slices in the axial plane at 2-mm intervals. During the study period, dose planning was accomplished using a Leksell Gamma knife C-model from 1995 to December 2010 and using a Leksell Gamma knife Perfexion™ (ELEKTA, Stockholm, Sweden) model after January 2011. After dose planning, GKRS was performed, and the mean treated tumor volume at the time of this procedure was 8185 mm3 (range 10–19500 mm3). In addition, the median dose delivered to the tumor margin was 25 Gy (range 10–30 Gy) with a median isodose line of 50% (range 40–75%).
We performed two GKRS treatments in eight patients who had a local recurrence of tumor or multiple new metastases. At the first session, the mean treated tumor volume and the median radiation dose were 6783 mm3 (range 10–14500 mm3) and 23 Gy (range 15–28 Gy), respectively. The median interval time between the two sessions was 4.5 months (range 1–18 months). At the second session, the mean treated tumor volume and the median radiation dose were 4248 mm3 (range 180–12600 mm3) and 20 Gy (range 14–25 Gy), respectively. All patients received methylprednisolone for up to 7 days to prevent acute brain swelling after GKRS. Follow-up imaging was performed 1–3 months after GKRS and every 3 months thereafter, depending on the patient's medical condition.
Radiological outcome evaluation
Tumor control was evaluated by measuring the tumor volume on MRI and comparing between pre-GKRS and follow-up MRI scans. The follow-up MRI was taken at 2–3 months after GKRS, and the response was classified as a complete response (CR, tumor disappearance), partial response (PR, ≥20% tumor volume reduction), stable disease (tumor volume reduction or enlargement <20%), or tumor progression (≥20% volume enlargement). A complete response, partial response, and stable disease were indicative of tumor control. To verify that an increase in the volume of the enhancing lesions on the 2nd follow-up MRI was due to a local recurrence, we performed methionine PET and/or perfusion MRI to discriminate tumor recurrence from radiation necrosis3839). Local recurrence was defined as any secondary increase in tumor volume that had initially responded to the GKRS, and distant recurrences were defined as any newly developed metastatic lesions remotely located from previously treated lesions. Overall survival time was defined as the interval between the first GKRS and death or the last follow-up examination.
Statistical analysis
Prognostic factors affecting local tumor control and survival were estimated using Kaplan-Meier survival plots. Univariate and multivariate predictors of survival were assessed via a Cox proportional hazards model. A p value<0.05 was considered statistically significant. Independent variables were tested categorically and included age (cutoff value of 40 years), KPS scores (cutoff value of 80), RTOG RPA class (III vs. II or I), number of brain metastases (cutoff value of 4), presence of pre-GKRS WBRT, presence of pre-GKRS tumor resection, presence of pre-GKRS cyst aspiration, total volume of the brain metastasis (<5000 mm3 or 5000–10000 mm3 vs. >10000 mm3), and radiation dose (cutoff value of 20 Gy). All statistical analyses were conducted using Statistical Product and Service Solution software version 21 (IBM Corp., Armonk, NY, USA).
RESULTS
Local tumor control
Of the 26 patients who were treated with GKRS during the study period, 24 cases (59 lesions) were available for a follow-up MRI, with two patients dying prior to follow-up due to systemic disease. The mean follow-up period for all patients was 21 months (range 1–102 months). The radiological response to GKRS was classified as one of four classes depending on the tumor volume between the pre-GKRS and follow-up MRI. On MRI, a complete response occurred in 39 lesions (66.1%), a partial response in 11 lesions (18.6%), and stable disease in 3 lesions (5.1%). These three groups were classified as controlled tumors (53 lesions with 89.8%). Another six lesions (10.2%) showed tumor progression with a 4-month median time to tumor progression. This indicates that the local tumor control rate was 89.8% (53 of 59 tumors).
Five patients who had six lesions with tumor progression all expired, with the causes of death including hydrocephalus due to cerebellar metastasis (4 months after GKRS), progression of leptomeningeal seeding (29 months after GKRS), multiple brain metastasis (3 months after GKRS), and respiratory failure due to a lung metastasis (2 and 5 months after GKRS).
Local and distant recurrences
A local recurrence was confirmed to have occurred in six patients with eight lesions (18.6%), with a median time of 11 months (range 2–29 months). Five of these patients (comprising six lesions) received an additional GKRS as a local treatment. The other patient could not receive further treatment owing to a generally poor condition. A distant recurrence occurred in five patients, with a median time of 9 months (range 2–28 months). In this group, four patients received an additional GKRS. The remaining patient could not due to a poor general condition. In this group of six local recurrences and five distant recurrences, one patient had both types of recurrence. There was no radiation necrosis in these patients. In summary, within the study period, eight patients underwent multiple GKRSs for local recurrence, distant recurrence, or both. The median interval between these GKRS treatments was 4.5 months. The median overall survival of these patients was 21 months (range 5–101 months) after the 1st GKRS, which was superior to the survival outcomes in our total study population [9.5 months (range 1–102 months)] after GKRS. The mean treated tumor volume in our cohort was 6782.5 cm3 at the 1st GKRS and 4247.5 cm3 at the 2nd GKRS. The median radiation dose was 22.6 Gy at the 1st GKRS and 20 Gy at the 2nd GKRS. In these patients, there was no side effect of repeated radiosurgery, such as radiation necrosis.
Overall survival and prognostic factors
Of the 24 patients included in our current series, six were alive at the end of the study. The overall survival rates were 82.1%, 34.7%, 13.0%, and 6.9% at 1, 6, 12, and 24 months after GKRS, respectively (Fig. 1). The median overall survival was 9.5 months after GKRS (range 1–102 months). The median overall survival for specific cancers after GKRS was 12 months (range 1–102 months) for ovarian cancer, 7.5 months (range 2–51 months) for endometrial cancer, and 3.5 months (range 1–18 months) for cervical cancer. That the cancer type is an important factor for overall survival was evident, but not statistically significant by univariate analysis [ovarian vs. endometrial, hazard risk (HR)=0.86, p=0.801; endometrial vs. cervical, HR=0.54, p=0.387; ovarian vs. cervical, HR=0.63, p=0.129] (Fig. 2).
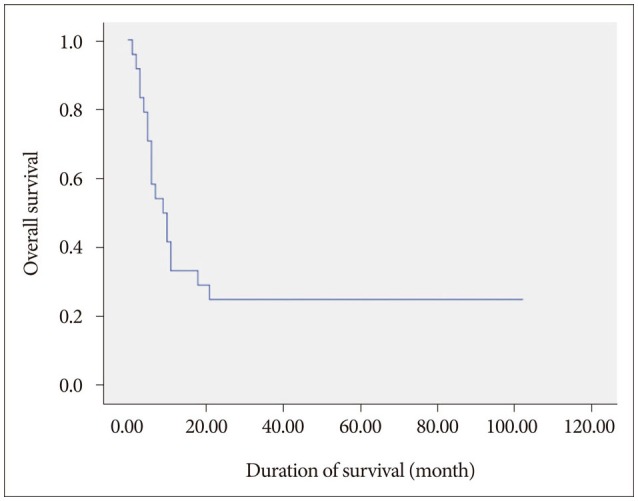
Kaplan-Meier curve of overall survival outcomes after GKRS. The median survival time after GKRS was 9.5 months. The survival rates were 82.1%, 34.7%, 13.0%, and 6.9% at 1, 6, 12, and 24 months after GKRS, respectively.
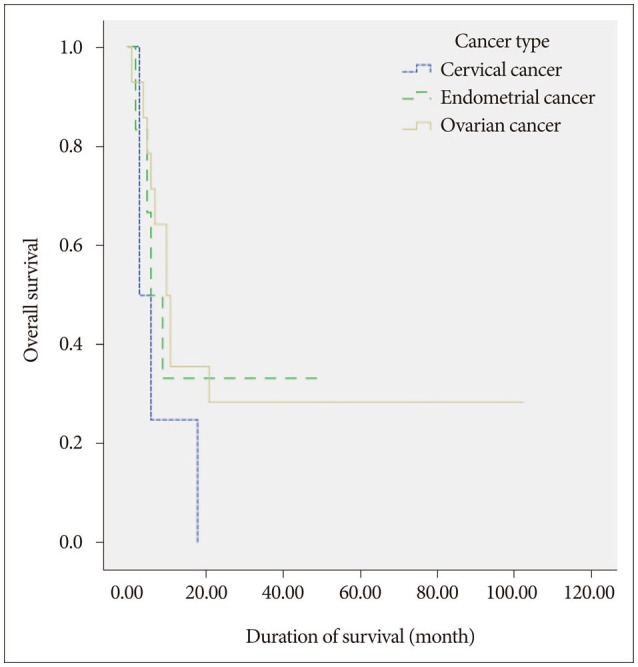
Kaplan-Meier curve of overall survival after treatment of ovarian, endometrial and cervical cancer brain metastasis with GKRS. Patients with ovarian cancer had a median survival of 12 months. Patients with endometrial cancer had a median survival of 7.5 months, and patients with cervical cancer had a median survival of 3.5 months. There was no statistical significance between the groups (ovarian cancer vs. endometrial cancer, p=0.801, endometrial cancer vs. cervical cancer, p=0.387, cervical cancer vs. ovarian cancer, p=0.129).
By univariate analysis, we found that age (≥40 years), KPS score (≥80), and the RPA class (class 1) were significant prognostic factors for overall survival (HR=0.176, p=0.008, HR=0.161, p=0.005, and HR=0.104, p=0.033, respectively) and the number of treated lesions (<4) was positively associated with overall survival (HR=2.752, p=0.052). Age-associated multivariate analysis indicated that the KPS score (≥80), the RPA class (class 1), and the number of treated lesions (<4) were significant prognostic factors for overall survival (HR=0.162, p=0.008, HR=0.107, p=0.038, and HR=2.897, p=0.045, respectively) (Table 2, Fig. 3).

A : Age-associated multivariate analysis indicated that the KPS score (≥80) was a significant prognostic factor for overall survival (HR=0.162, p=0.008). B : RPA Kaplan-Meier survival curve showed significant difference between the three classes (Class 2, HR=0.508, p=0.178, Class 1, HR=0.107, p=0.038). C : Age-associated multivariate analysis showed that the number of treated lesions (<4) was a significant prognostic factor for overall survival (HR=2.897, p=0.045).
DISCUSSION
Brain metastases from gynecologic cancers
Brain metastases represent the most common type of intracranial malignancy33). Lung cancer, renal cell carcinoma, breast cancer, and melanoma represent the majority of cases of metastatic brain tumors, and their surgical and adjuvant treatments are well described2510). Brain metastases from gynecologic cancers are very rare, but appear to have increased in incidence in recent years due to improved survival14172325). Increased survival may be due to improved treatment modalities; 39% of patients with brain metastases are heavily treated with three or more lines of chemotherapy15), and increased diagnostic sensitivity resulting from improved cerebral imaging technology has made detecting small intracranial lesions at an earlier stage of disease recurrence possible13). The prognosis of brain metastasis from gynecologic cancers is generally poor. Cohen et al.7) reported 72 cases of brain metastases from ovarian and endometrial cancer with median survival of 6–7 months and 1–2 months after the diagnosis of brain metastases, respectively2123). Nasu et al.23) reported a median survival of 5 months following the diagnosis of intracranial metastatic cervical cancer.
GKRS as novel alternative to surgery
In our current cases, a total of 24 patients with gynecologic cancer underwent GKRS for a brain metastasis. Fourteen of these patients had brain metastases from ovarian cancer with a median survival of 14.5 months, and six patients had brain metastases from endometrial cancer with a 7.5-month median survival and four patients had brain metastases from cervical cancer with a median survival of 5.5 months after the diagnosis of brain metastasis. And the median survival after GKRS was 12 months for ovarian cancer, 7.5 months for endometrial cancer, and 3.5 months for cervical cancer. This suggests that GKRS is an effective treatment modality for a brain metastasis from a gynecologic cancer. Differences in survival may be related to several factors. First, early detection and early treatment may affect the overall survival; with advances in MRI, small lesions can be detected and treated early which may improve survival outcomes4). In addition, the development of treatment modalities with multidisciplinary approaches may contribute to an improved overall survival16). Traditionally, WBRT has been a widely used treatment for brain metastasis. At this time, however, GKRS is also a suitable alternative to surgery combined with WBRT34). The current management of advanced stage ovarian cancer includes cytoreductive surgery followed by adjuvant chemotherapy3). Some reports have shown that radiation therapy combined with surgery or chemotherapy is superior to radiation alone712). Since radiation therapy can be applied instead of surgery to most patients with poor health conditions and inaccessible or multiple lesions, it has been the standard intervention for brain metastases for several decades16). Recently, stereotactic radiosurgery including GKRS has been reported to be as effective as open surgery for local tumor control2235). As a result, GKRS is being considered as an alternative to surgical resection for small metastases without a mass effect. GKRS can be used for patients with tumors in or near the eloquent cortex, with deep lesions, or at high risk from anesthesia40).
Advantage of GKRS
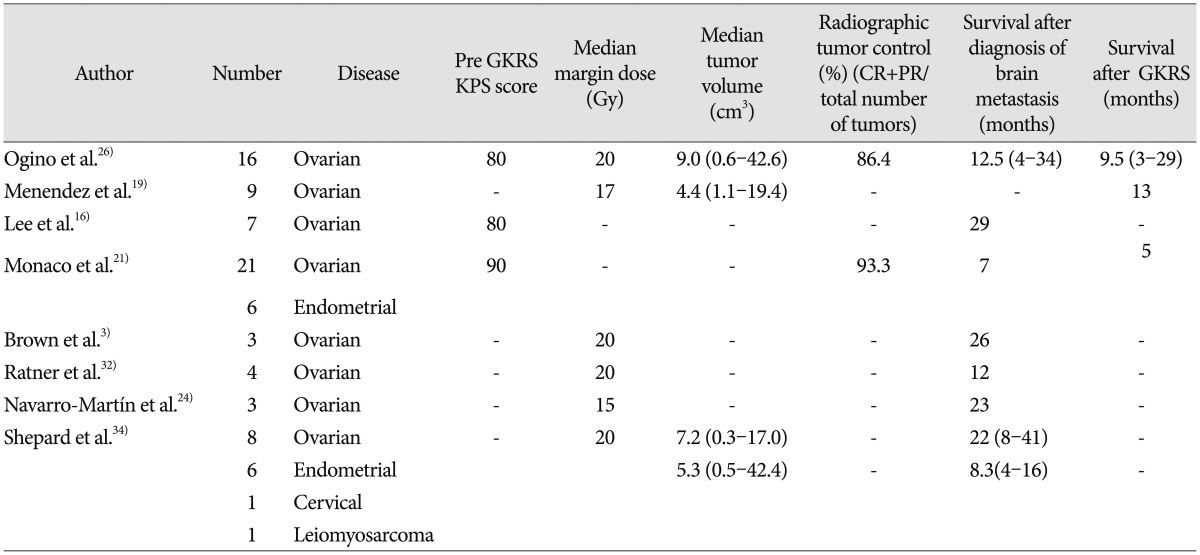
GKRS has proven to be an effective therapeutic modality for the treatment of smaller solitary and multiple brain metastases because of its reduced invasiveness and its ability to target surgically inaccessible lesions and achieve local tumor control21). Furthermore, although there have been no direct randomized clinical comparisons of GKRS with other surgical-radiation protocols, the results we obtained in our current patients with GKRS were similar or superior to those obtained using other methods112228). Some earlier studies have reported the utility of GKRS for the treatment of brain metastases from gynecologic cancers (Table 3)316192124263234). The median survival time after GKRS for patients with a brain metastasis from a gynecologic cancer ranged from 5–13 months in those studies, which is similar to the median survival time of 9.5 months in our current patients. These data collectively confirm the utility of GKRS as a treatment modality for a brain metastasis from a gynecologic cancer. Additionally, Corn et al.9), have reported that GKRS produced more frequent complete radiographic responses in brain metastases from ovarian cancer (40% vs. 29%) and higher 2-year survival rates (60% vs. 15%) compared with WBRT. Therefore, novel procedures like GKRS have been employed in an attempt to improve survival.
Prognostic factors for the GKRS
However, the follow-up period after GKRS remains relatively short, and the number of patients who have undergone this procedure is still low. Understanding the prognostic indicators for GKRS for the gynecologic cancers is therefore important. Previous studies have reported that an improved performance status, single brain metastasis, and the absence of extracranial disease are associated with improved outcomes for patients with brain metastasis from gynecologic malignancies23). With regards to endometrial cancer, three factors–high tumor grade, advanced stage of disease, and the presence of lymphovascular invasion–have been reported to be correlated with the future development of a brain metastasis21). In our present analysis, an older age (≥40 years), elevated KPS score (≥80), low RPA class (class 1), and small number of treated lesions (<4) were found to be associated with improved survival outcomes in our patients with brain metastasis from a gynecologic cancer. In particular, we found that older patients may have better survival because younger patients with a brain metastasis appear have a more aggressive primary cancer. Although cancer type is generally an important factor influencing overall survival1423), we found no statistical differences between the different gynecologic cancer types in our current study, perhaps because the number of patients in our series was insufficient. We speculate therefore that the differences in survival between different primary cancers after GKRS are due to variations in the clinical course of the primary disease.
Follow-up after GKRS
MRI was used for routine follow-up in our series. However, when the interpretation between radiation necrosis and tumor recurrence was difficult on the MRI scan, a methionine PET scan was used to differentiate tumor recurrence from radiation necrosis. Methionine PET proved to be an effective method to discriminate a tumor recurrence from radiation necrosis20), but defined cut-off values remain to be determined and vary among previous studies. Further research is needed to utilize methionine PET as an efficient tool for differentiation. When a follow-up image showed a local recurrence or distant metastases, an additional GKRS was considered as part of the treatment for our patients. In our present study cohort, eight patients received repeated GKRS treatments due to a local recurrence, distant recurrence, or both. None of our current study patients developed a radiation-induced morbidity.
Limitation of the study
The major limitations of this study included its retrospective nature and the relatively small number of patients we examined. An uncharacterized chemotherapy regimen and heterogeneity of systemic conditions were also potential limitations of our analysis. However, our findings indicate that GKRS is a good choice of treatment for a metastatic brain tumor from a gynecologic cancer, and our findings warrant further verification.
CONCLUSION
Metastatic brain tumors from gynecologic cancers are rare but have increased in frequency in recent years. GKRS has proven to be an effective and safe treatment modality. In these cases with an older age (≥40 years), high KPS score (≥80), low RPA class (class 1), and a lower number of treated lesions (<4) associated with better survival.
Acknowledgements
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, & Future Planning (NRF-2010-0019351).