Assessing the Adequacy of Superficial Temporal Artery Blood Flow in Korean Patients Undergoing STA-MCA Anastomosis
Article information
Abstract
Objective
Superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis is conducted for flow augmentation. In this study, we measured the STA cut flow of a Korean population and evaluated the relationship between STA cut flow and long-term patency of the bypass.
Methods
A retrospective study was conducted. Intraoperative measurement of STA flow was conducted using a microvascular flow meter on patients who underwent STA-MCA. After cutting the distal end, the STA flow rate was measured with no resistance and recorded. After finishing anastomosis, STA flow was measured and recorded. The cut flow index was calculated by dividing post anastomosis flow by cut flow in intracranial atherosclerotic stenosis patients.
Results
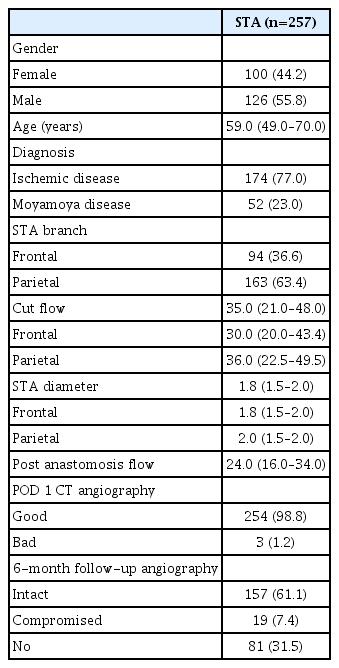
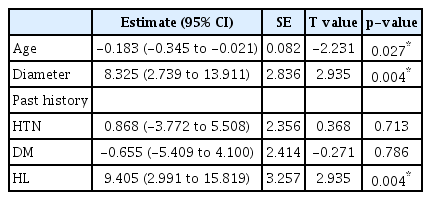
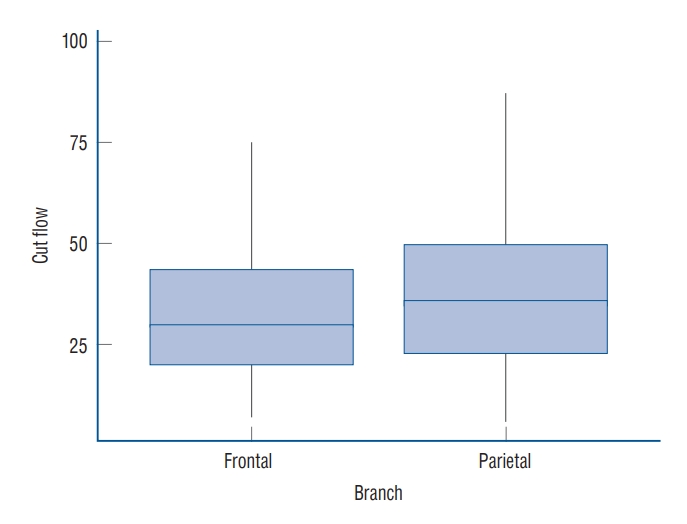
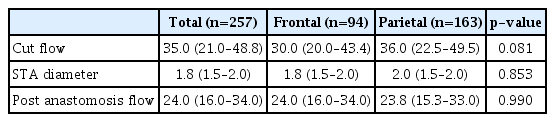
The median STA cut flow was 35.0 mL/min and the post anastomosis flow was 24.0 mL/min. The cut flow of STA decreased with aging (p=0.027) and increased with diameter (p=0.004). The cut flow showed no correlation with history of hypertension or diabetes mellitus (p=0.713 and p=0.786), but did correlate a positively with history of hyperlipidemia (p=0.004). There were no statistical differences in cut flow, STA diameter, and post anastomosis flow between the frontal and parietal branches (p=0.081, p=0.853, and p=0.990, respectively).
Conclusion
The median STA cut flow of a Korean population was 35 mL/min. Upon reviewing previous articles, it appears that there are differences in the STA cut flow between Western and Asian patients.
INTRODUCTION
Superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis is a surgical procedure performed as a countermeasure for hypoperfusion or parent vessel occlusion due to aneurysm trapping [3,11]. This is considered a low-flow bypass technique that utilizes a STA with a diameter of around 2 mm. After the STA is trimmed, the initial flow is very slow but gradually increases over time [1]. Manipulation of the vessel during harvesting can cause vasoconstriction, which can be relieved by applying papaverine and allowing the flow to increase slowly. In some cases, the STA may be damaged during harvesting, in which case another donor vessel should be used for a successful bypass. Therefore, the surgeon should have a good understanding of the STA cut flow to determine if the blood flow is within a normal range. According to anecdotal evidence, the STA f low is between 15–25 mL/min among Western patients [6], however, there is limited data available and no clear information about the Asian population. This study aims to introduce surgical techniques that maximize STA flow and to identify reference values for blood flow increase after cutting the distal end in Korean patients. The minimum and maximum cut flow values of the STA were measured directly during surgery.
MATERIALS AND METHODS
The Institutional Review Board of The Catholic University of Korea approved the current study (approval No. HC23RI-SI0019).
A retrospective study was conducted on patients aged between 20 and 80 years who underwent STA-MCA anastomosis for intracranial atherosclerotic stenosis (ICAS) or Moyamoya disease (MMD) from May 2012 to October 2021. The patients were planned to undergo bypass surgery using preoperative magnetic resonance imaging (MRI), magnetic resonance angiography, perfusion MRI, single photon emission computed tomography (SPECT), and digital subtraction angiography (DSA). The indications for surgery were 1) acute cerebral infarction due to large cerebral artery occlusion, 2) a severe perfusion defect, e.g., with a T max 6 volume of 70 mL more and ineligibility for intra-arterial thrombectomy in ICAS patients and decreased reserve capacity from Diamox SPECT or a high risk of rebleeding in patients with MMD. The donor vessel was determined by DSA before surgery. In the case of a single bypass, the parietal branch of the STA was selected first. In the case of a double barrel, the frontal branch and parietal branch were harvested in turn.
Operative method
After assessing the subcutaneous course of the STA using Doppler imaging, skin marking and draping were completed, then, 0.5% lidocaine was injected into the perivascular galea to assist with STA dissection. A skin incision was made using a No. 15 surgical blade, and the vascularized galea flap was harvested using a Metzenbaum scissors. After harvesting the STA, the distal area of the vessel was cut and trimmed to match the anastomosis site.
The cut flow was measured using a microvascular ultrasonic flowmeter (Charbel micro-flow probe; Transomics Systems, Inc., Ithaca, NY, USA), while the temporary clip of the proximal STA was opened. Immediately after harvesting the vessel, blood flow was ≤5 mL/min. At this time, cotton soaked with papaverine was applied to the blood vessel and the cut flow was measured at 5-minute intervals while waiting for resolution of the vasoconstriction that occurred during harvest. Measurements were repeated until they were increased and remained constant. If the change was ≤2 mL/min, it was recorded as the patient’s final STA cut flow. Conversely, if the blood flow did not increase even after these attempts, further release of fibrous soft tissue covering the STA and papaverine administration were completed. The cut flow was measured at 5-minute intervals to increase the cut flow to ≤2 mL/min in the same way (Fig. 1). Thereafter, STA-MCA anastomosis was performed in the usual manner. After anastomosis, blood flow was measured through intraoperative micro-Doppler (Nicolet Biomedical, Madison, WI, USA) and patency was confirmed with an indocyanine green angiogram. Then, donor vessel flow was measured using a flowmeter and recorded as post-anastomosis flow. The ratio of cut flow to post anastomosis flow was calculated and recorded as the cut flow index (CFI). In cases with intraoperative CFI ≤0.5, an additional bypass procedure using another STA branch was performed for successful revascularization. The diameters of the donor and recipient vessels were measured using a micro-gauge. STA patency was confirmed through computed tomography (CT) angiography 6 hours after the surgery, and the final STA patency was evaluated by DSA after 6 months. Cases with good intracranial flow irrigation through the STA as confirmed by DSA were classified into the intact STA group, while those with occluded or weak intracranial flow were classified as the compromised STA group.
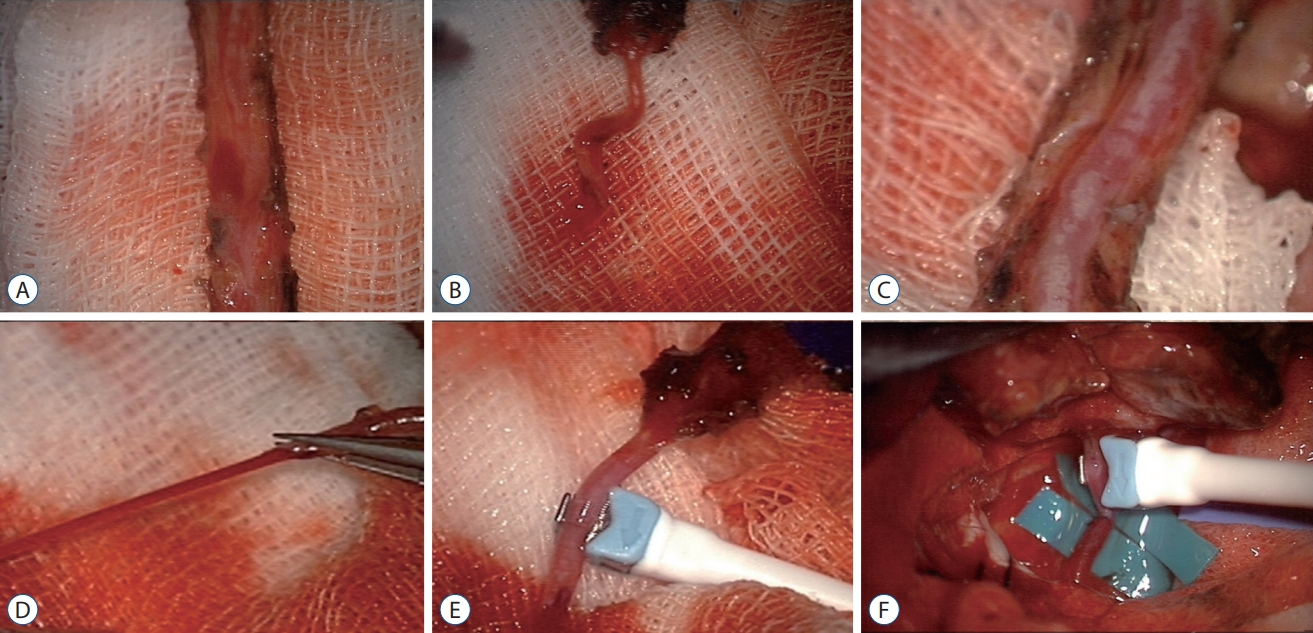
Operative method of superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis. A : The vascularized galeal flap was harvested using a Metzenbaum scissors. B : Immediately after harvest, blood flow is low. C : Removing fibrous soft tissue covering the STA. D : After removing fibrous soft tissue covering the STA and applying papaverine, the cut flow increased gradually. E : Using microvascular flow-meter, cut flow of STA was measured. F : End-to-side anastomosis of STA-MCA was performed.
Statistical method
Patient characteristics were described using the median frequency and interquartile range. Linear regression was used to analyze the association between cut flow and patient factors. A generalized linear model was employed to compare the variables of the frontal and parietal branches that underwent STA-MCA anastomosis. The Wilcoxon rank-sum test was applied to compare variables between the STA frontal branch and parietal branches, as well as to determine long-term STA patency at 6 months after anastomosis. The impact of hyperlipidemia was analyzed using analysis of variance. All p-values were 2-sided, and p-values ≤0.05 was considered significant. R software (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
RESULTS
A total of 226 patients underwent STA-MCA anastomosis, when, including double-barrel cases, a total of 257 STAs was analyzed. The median age was 59.0 years (48.5–70.0). One hundred seventy-four patients had ICAS, while 52 had MMD (hemorrhagic or ischemic type). Totals of 94 frontal branches and 163 parietal branches were measured. In three cases, the flow was ≤10 mL/min and did not increase with any management. If donor vessel damage (e.g., intraluminal dissection of a partial tear) was confirmed, the damaged area was cut, and end-to-end anastomosis was performed to maintain flow. The cut flow, which was measured immediately after trimming the STA, was about 7–25 mL/min. One hundred twenty-nine cases showed a gradual increase in STA cut flow after applying papaverine. One hundred twenty-eight cases did not show an increase after applying papaverine alone and required release of perivascular fibrous tissue surrounding the STA. Releasing perivascular fibrous tissue took an average of 10 minutes, and stable cut flow was maintained within 5–10 minutes after releasing the tissue (Fig. 2).
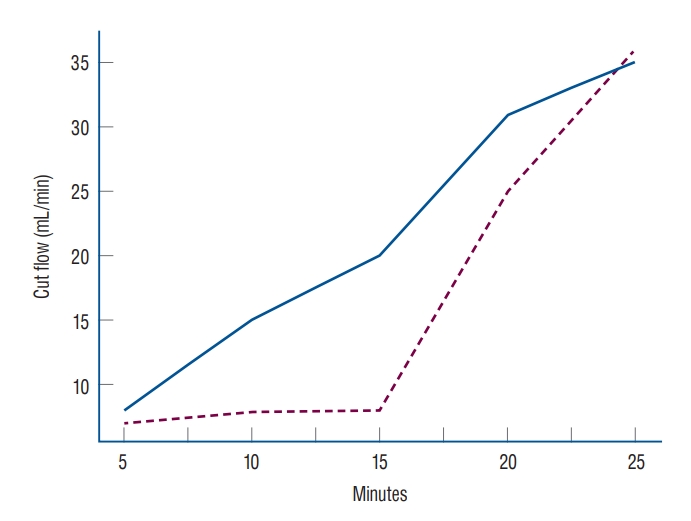
The cut flow value of superficial temporal artery (STA) over time. One hundred thirty cases showed a gradual increase in STA cut flow after application of papaverine (blue). One hundred twenty-eight cases required release of perivascular fibrous tissue surrounding the STA. Release of the perivascular soft tissue took an average of 10 minutes (dashed).
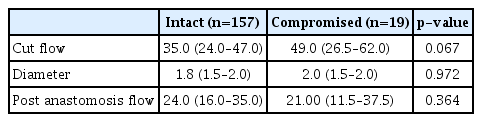
The median cut flow of the whole case study population was 35.0 mL/min (6.0–98.0), while the median STA diameter was 1.8 mm (0.7–3.0), with median diameters of the frontal and parietal branches of 1.8, 2.0 mm, respectively. The median post-anastomosis flow measured using the flow meter was 24.0 mL/min (2.0–81.0). According to brain CT angiography performed 6 hours after the surgery, STA patency was intact in 254 cases, while flow was not identified in three cases. Of the total 257 bypasses performed in this study, follow-up angiography at 6 months confirmed persistency in 176 cases. In total, 157 bypass flows remained intact and were classified as the STA intact group (Table 1).
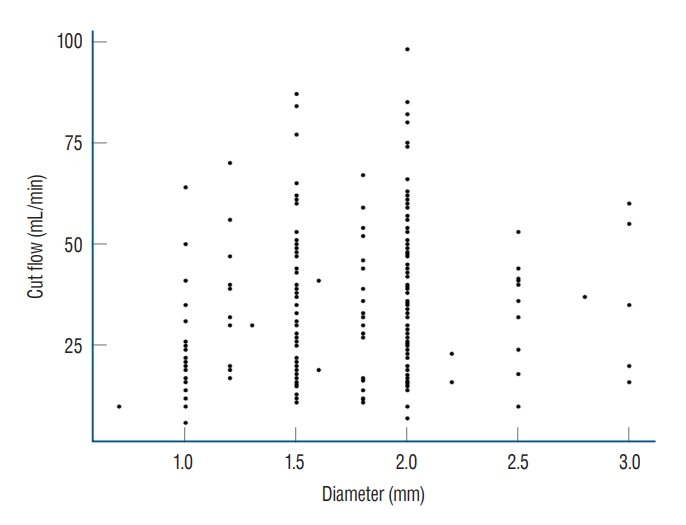
Factors affecting cut flow were analyzed through multivariate analysis. As patient age increased, cut flow decreased significantly (p=0.027), as vessel diameter increased, cut flow increased significantly (p=0.004, Fig. 3). There was no significant difference in cut flow according to history of hypertension or diabetes mellitus (p=0.713 and p=0.786, respectively), but patients with hyperlipidemia presented higher cut flow values (p=0.004, Table 2). In the analysis conducted by dividing STA diameter using a 2 mm threshold, it has been observed that the presence of hyperlipidemia still inf luences the f low (p=0.013, Table 3).
Analysis was performed to assess the difference between the frontal branch and the parietal branch. The median cut flow of the parietal branch was 36 mL/min, while that of the frontal branch was 30 mL/min, showing no significant difference (p=0.081, Fig. 4). The median STA diameter was 1.8 mm in the frontal branch and 2.0 mm in the parietal branch, with no significant difference (p=0.853). Finally, there was no significant difference in post-anastomosis flow between the frontal and parietal branches (p=0.990) (Table 4).
Further analysis of 157 cases with intact STA flow (intact group) and 19 cases with poor flow or occlusion (compromised group) on follow-up angiography was performed. The cut flow values displayed a non-significant difference between the groups, with median of 35.0 mL/min (7.1–85.0) in the intact group and 49.0 mL/min (16.4–98.0) in the compromised group (p=0.067). There was also no significant difference in diameter of STA, with a median of 1.8 mm (0.7–3.0) for the intact group versus that of 2.0 mm (1.0–2.0) in the compromised group (p=0.972). The median post-anastomosis flow was 24.0 mL/min in the intact group and 21.0 mL/min in the compromised group, with no statistical difference (p=0.364) (Table 5).
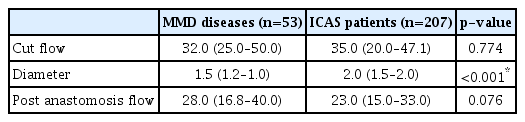
Additional analyses for patients with MMD and ICAS were done. The cut flow between the two groups did not show a significant difference, with median values of 32.0 mL/min and 35.0 mL/min, respectively (p=0.774). However, the diameter was found to be significantly larger in the ICAS group (p<0.001). Regarding post anastomosis flow, no significant difference was observed between the two groups (p=0.076) (Table 6). For ICAS patients, we conducted an analysis of the CFI, which showed a median value of 0.64 (0.47–1.05) with a minimum of 0.06 and a maximum of 4.00. Regarding the long-term follow-up flow, CFI demonstrated borderline significance (p=0.063).
DISCUSSION
STA-MCA bypass is a low flow bypass procedure that is used for flow augmentation during intentional parent vessel occlusion for managing a large cerebral aneurysm or in cases of severe perfusion defects in patients with MMD or ICAS [3,10,11]. However, there are few quantitative investigations of the amount of blood supplied into the cranial cavity through an STA with about 2 mm in diameter. Cut flow is the blood flow measured through the dissected STA after cutting the distal end during anastomosis. Although it is not possible to confirm a exact value, low flow bypass refers to a flow ≤50 mL/min, and high flow bypass refers to a flow ≥50 mL/min [9]. In STA-MCA anastomosis, the flow is usually around 15–20 mL/min [6]. Conversely, in high flow graft, a flow of about 70–140 mL/min is supplied [8,13]. However, there is insufficient numerical data on these measures. According to our study, which investigated a Korean population, patients showed a median STA cut flow of 35 mL/min (6–98), and 18.6% of patients displayed a flow ≥50 mL/min. This analysis revealed that STA can supply more blood than expected.
The relationship between cut flow and diameter according to Poiseuille’s equation is that blood flow rate increases as the force of the vessel wall that causes resistance to blood flow in the blood vessel decreases [4] Therefore, the larger is the diameter, the higher is the cut flow (p=0.004). One could predict the flow through the larger diameter among the branches of STA, which indicates that a high cut flow can be obtained. Analysis of factors affecting cut flow showed that cut flow decreased with age (p=0.027). Neither a history of hypertension nor one of diabetes mellitus had a significant effect on STA cut flow (p=0.713 and p=0.786, respectively), but patients with hyperlipidemia had a higher cut flow than those without (p=0.004). Even considering effect of vessel diameter on cut flow, history of hyperlipidemia showed positive effect on cut f low (p=0.007). On the other hand, previous studies contend that hyperlipidemia is associated with a reduction in cerebral blood flow due to effects on blood viscosity or the vessel itself, and hyperlipidemia is an important factor in atherosclerosis [2,16]. The higher STA cut flow in patients with hyperlipidemia in this study suggest that hyperlipidemia has different tendencies in its effect in intracranial vessels and extracranial vessels, and additional research could be planned.
When the difference between 94 frontal branches and 163 parietal branches was analyzed, the cut flow of the parietal branch showed no difference compared to that of the frontal branch (p=0.081). The median STA diameter in the frontal branch was 1.8 mm and that in the parietal branch was 2.0 mm, while the median post anastomosis flow was 24.0 mL/min in the frontal branch and 23.80 mL/min in the parietal branch, without a significant difference (p=0.990).
However, it was confirmed by 6-month follow-up angiography that there is no significant difference in STA cut flow between the intact and compromised group (p=0.067), and post anastomosis flow showed no difference between these groups (p=0.364). If post-anastomosis flow is maintained, it can be interpreted as securing long-term patency. In 14 of 19 poor or occluded cases, recanalization of the parent vessel occurred. In five cases, the flow through the STA was weak, but the collateral flow through the posterior cerebral artery or anterior cerebral artery was increased at long-term follow up angiography.
An absolute comparison between patients was not possible because samples could not be extracted under the same conditions. However, comparison of the cut flow values of Western studies with those of a study from China and this study suggests that the cut flow values in Eastern studies tend to be lower than those of Western populations [1,12,15] (Table 7). There was a scatterplot of Western patients in the article of Amin-Hanjani et al. [1] and the scatterplot of Korean patients was made under our data in the same format. Highest cut flow is significantly low in our data (Fig. 3). In a study analyzing the effect of ethnicity on the diameter of the carotid artery, the Hispanic group had a significantly smaller artery diameter but greater stiffness than the Caucasian group [7], and it is reasonable that there would be ethnic differences in cut flow, diameter, and post anastomosis flow of the STA. Also, there were differences in inclusion criteria between Western and Asian studies. Western studies only included patients with ischemic disease [1,12], but the study from China and our study included patients with ischemic disease or MMD [15]. Previous studies have analyzed differences in clinical features and incidence rates of MMD according to ethnicity [5,14]. These ethnic differences may affect not only the occurrence of MMD, but also, the clinical features of cranial arteries, including the STA.
In institutions without ultrasonic flowmeters for measuring cut flow, numerical evaluation of STA cut flow is possible by measuring the amount of blood dropped into a bottle for 1 minute after trimming the STA. This method enables a more quantitative approach to STA-MCA anastomosis and will help improve the surgical outcome.
CONCLUSION
This study analyzed STA flow, post anastomosis flow, and CFI in Korean patients using a total of 257 STAs that underwent STA-MCA bypass. The median STA cut flow of Koreans was 35 mL/min. After reviewing previous articles, it seems there are differences in the STA cut flow observed between Western and Asian patients.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : ISP; Data curation : ISP; Formal analysis : JE; Methodology : JE, ISP; Project administration : JE, ISP; Visualization : JE; Writing - original draft : JE, ISP; Writing - review & editing : JE, ISP
Data sharing
None
Preprint
None