Impact of Collateral Circulation on Futile Endovascular Thrombectomy in Acute Anterior Circulation Ischemic Stroke
Article information
Abstract
Objective
Collateral circulation is associated with the differential treatment effect of endovascular thrombectomy (EVT) in acute ischemic stroke. We aimed to verify the ability of the collateral map to predict futile EVT in patients with acute anterior circulation ischemic stroke.
Methods
This secondary analysis of a prospective observational study included data from participants underwent EVT for acute ischemic stroke due to occlusion of the internal carotid artery and/or the middle cerebral artery within 8 hours of symptom onset. Multiple logistic regression analyses were conducted to identify independent predictors of futile recanalization (modified Rankin scale score at 90 days of 4–6 despite of successful reperfusion).
Results
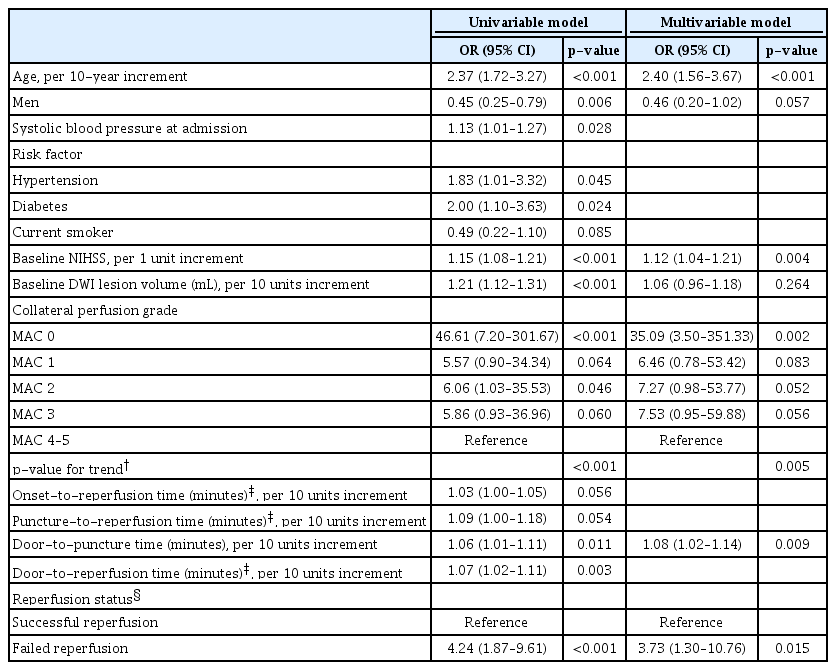
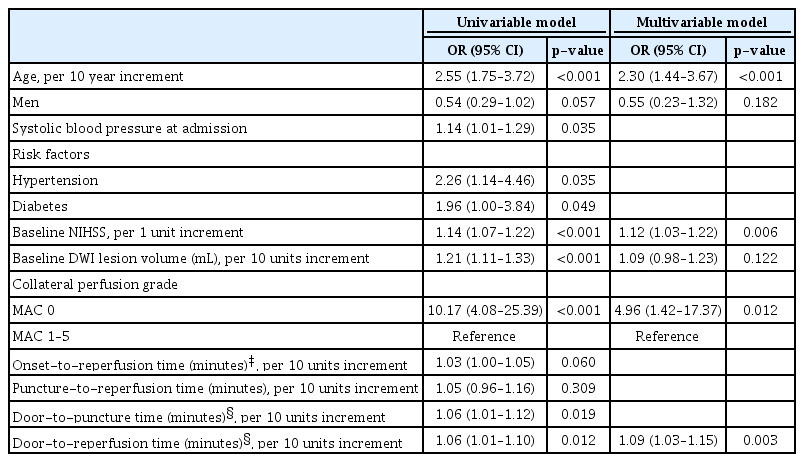
In a total of 214 participants, older age (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.56 to 3.67; p<0.001), higher baseline National Institutes of Health Stroke Scale (NIHSS) scores (OR, 1.12; 95% CI, 1.04 to 1.21; p=0.004), very poor collateral perfusion grade (OR, 35.09; 95% CI, 3.50 to 351.33; p=0.002), longer door-to-puncture time (OR, 1.08; 95% CI, 1.02 to 1.14; p=0.009), and failed reperfusion (OR, 3.73; 95% CI, 1.30 to 10.76; p=0.015) were associated with unfavorable functional outcomes. In 184 participants who achieved successful reperfusion, older age (OR, 2.30; 95% CI, 1.44 to 3.67; p<0.001), higher baseline NIHSS scores (OR, 1.12; 95% CI, 1.03 to 1.22; p=0.006), very poor collateral perfusion grade (OR, 4.96; 95% CI, 1.42 to 17.37; p=0.012), and longer door-to-reperfusion time (OR, 1.09; 95% CI, 1.03 to 1.15; p=0.003) were associated with unfavorable functional outcomes.
Conclusion
The assessment of collateral perfusion status using the collateral map can predict futile EVT, which may help select ineligible patients for EVT, thereby potentially reducing the rate of futile EVT.
INTRODUCTION
In recent randomized clinical trials, endovascular thrombectomy (EVT) has been proven to have considerable therapeutic effects in patients with acute ischemic stroke due to large vessel occlusion [4]. However, a substantial proportion of patients experience futile reperfusion, defined as a poor long-term outcome despite successful reperfusion. A meta-analysis that pooled the results of five randomized clinical trials showed that poor outcome (modified Rankin scale [mRS] score at 90 days of 3–6) occurs in 54% of cases with a Thrombolysis in cerebral infarction (TICI) score of 2b/3 [4]. The primary goal of reperfusion treatments is to save the salvageable brain to improve functional outcomes. The high rate of futile reperfusion reflects that patients who were unlikely to benefit from reperfusion treatments because they had a minimal amount of salvageable brain were included in current EVT.
Cell viability and functional outcomes in patients with acute ischemic stroke caused by proximal arterial occlusion mainly depend on collateral circulation [14,23]. Previous studies have shown that good collaterals slow infarct growth and that poor collaterals accelerate it. Therefore, better collaterals are associated with less infarct growth and better functional outcomes, while poor collaterals are linked to unfavorable functional outcomes even after successful reperfusion [12,13,15,27]. Collateral status can be a direct predictor of futile reperfusion. Recent studies have shown that poor collateral status may predict futile reperfusion [17,26]. However, there is clinical uncertainty in the guidelines for EVT decisions regarding the specific criteria for the collateral circulation status. The imaging methods used in previous studies have major problems in collateral estimation. The arterial scoring method based on single-phase computed tomography (CT) angiography had a critical limitation of underestimating leptomeningeal collaterals with a longer transit time due to early triggering of static acquisition [18]. A method based on multiphase CT angiography overcame this limitation but still has the problem that the phases are determined not by the patient’s hemodynamics but by the table speed of the CT machine; therefore, it can be inaccurate in individual patients [16].
Researchers have developed a multiphase collateral imaging method, known as the “collateral map”, and an accompanying collateral perfusion grading method [21]. The collateral map is composed of the images of arterial, capillary, early venous, late enous, and delay phases, which are divided according to the patient’s hemodynamic status. The clinical value of the collateral map was supported by demonstrating linear associations between the collateral perfusion grades of the collateral map and functional outcomes in patients with acute ischemic stroke due to occlusion of the proximal anterior circulation [7]. The current study evaluated the role of the collateral map for the prediction of futile EVT in patients with acute ischemic stroke due to large-vessel occlusion in the anterior circulation.
MATERIALS AND METHODS
The Local Institutional Review Boards of Konkuk Medical Center and Daejeon St. Mary’s Hospital approved this study (IRB No. KUMC0114-01-100-007, DC21DIDI0050), and written informed consent was obtained from all participants.
Study participants
For this secondary analysis, we selected participants from the ongoing Database of Acute ischemic Stroke Analysis Network (DASAN) which is to investigate the optimal evaluation method in patients with acute ischemic stroke. The database contains clinical and imaging data of participants with acute ischemic stroke due to large vessel steno-occlusion. The data were prospectively collected from two university hospitals, commencing on January 1, 2016. The inclusion criteria of the DASAN were as follows : 1) participants older than 18 years of age; 2) participants with acute ischemic stroke due to occlusion or severe stenosis of the internal carotid artery and/or M1 or M2 segment of the middle cerebral artery, or the basilar artery, and 3) participants who underwent brain CT and magnetic resonance imaging (MRI) including diffusion-weighted imaging (DWI), susceptibility weighted imaging, dynamic contrast-enhanced MR angiography, dynamic susceptibility MR perfusion, and fluid attenuated inversion recovery at admission. The inclusion criteria for this study were as follows : 1) participants who were treated with EVT for acute ischemic stroke due to occlusion of the internal carotid artery and/or M1 or M2 segment of the middle cerebral artery and associated symptoms who were evaluated within 8 hours of symptom onset; or 2) participants who had a DWI lesion and no visible signal change on fluid attenuated inversion recovery in wake-up stroke or unclear onset >8 hours from last known well. The exclusion criteria were patients who had the following : occlusion of posterior circulation, follow-up loss at 3 months, a mRS score >2 at baseline, death related to procedural complications or other causes except stroke such as pneumonia, cancer, etc., recurrent acute ischemic stroke within 90 days, and uninterpretable poor quality collateral map.
Participants were evaluated for demographic data, medical history, vascular risk factors, routine blood test results, brain imaging, and cardiologic tests. The severity of stroke was assessed using the National Institutes of Health Stroke Scale (NIHSS). All participants underwent EVT, or intravenous thrombolysis followed by EVT. Reperfusion was assessed on the basis of conventional cerebral angiograms obtained at the end of the intra-arterial procedure and classified according to the modified TICI system as follows : 0, no reperfusion; 1, penetration with minimal reperfusion; 2a, <50% reperfusion; 2b, ≥50% reperfusion; 2c, near-complete reperfusion; and 3, complete reperfusion of the affected vascular territory [3]. Reperfusion status was classified as either successful reperfusion (modified TICI score of 2b to 3) or failed reperfusion (modified TICI score of 0 to 2a). Functional outcomes were assessed on day 90 using the mRS; unfavorable functional outcome was defined as a mRS score of ≥4 on day 90, which was considered an indicator of futile EVT in this study that can be excluded from the EVT [28]. Eighty-nine participants of the DASAN have been previously reported [7,11,12,21]. Previous articles dealt with the predictability of the collateral map or susceptibility-weighted imaging for functional outcomes or hemorrhagic transformation of participants with acute ischemic stroke, whereas in this manuscript, we report on futile EVT.
Imaging postprocessing and analysis
MRI was performed using 3-Tesla MRI machines (Magnetom Skyra [Siemens Healthcare, Erlangen, Germany] and Ingenia [Philips Healthcare, Best, Netherlands]). The acquisition parameters were the same as those in a previous study [21]. Using source data from dynamic contrast-enhanced MR angiography, 3-dimensional rotational arteriography was performed to determine the arterial status, and a multiphase MR angiography collateral map was reconstructed to evaluate the collateral perfusion status (Fig. 1). We generated the multiphase MR angiography collateral map by using an in-house program, which was composed of images in the arterial, capillary, early venous, late venous, and delay phases. The phases of the collateral map were automatically divided based on arterial and venous signal intensity-time curves obtained from region of interests on the normal side middle cerebral artery (MCA) and the superior sagittal sinus. The details of the methodology used were the same as those used in previous studies [21].
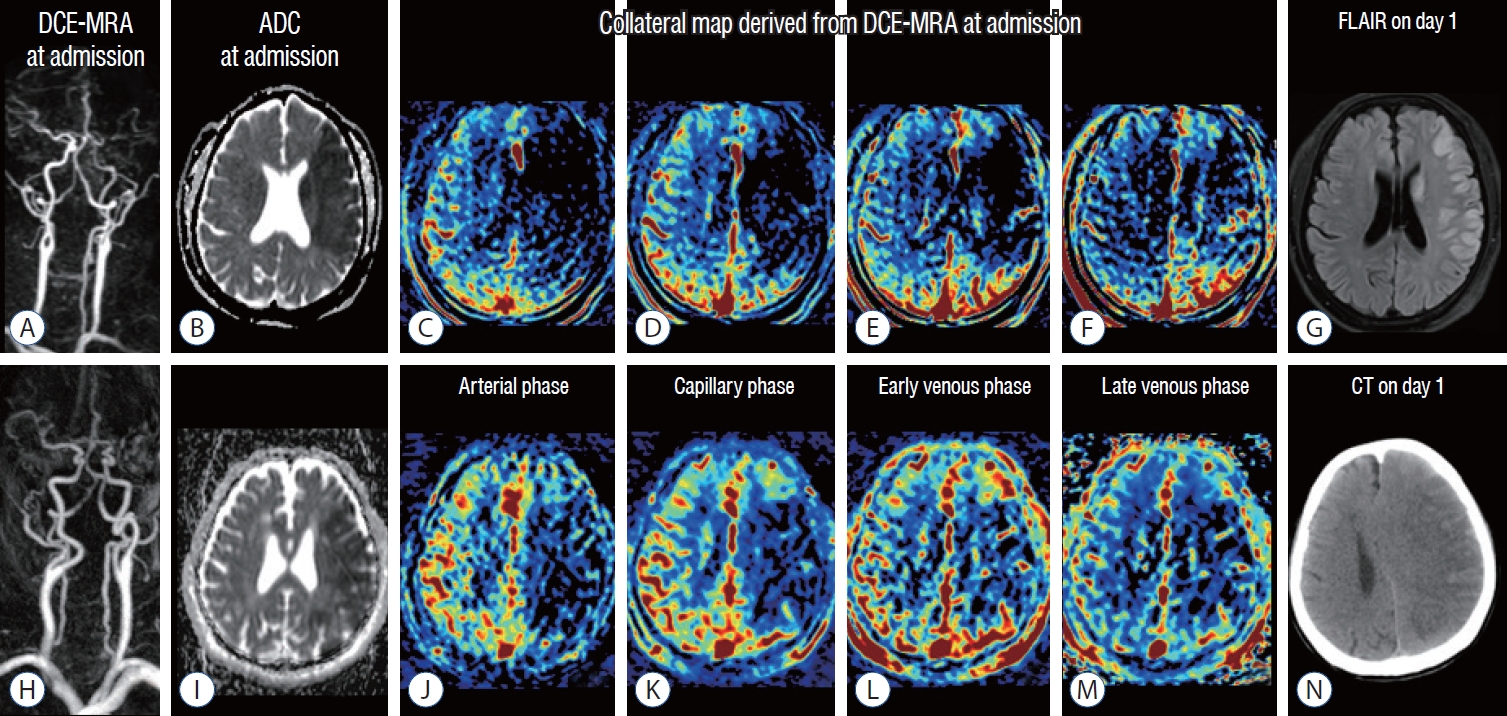
images of a man in his 50s (A-g) and a man in his 80s (h-n). The premorbid modified Rankin scale (mRS) score was 0 in both patients, and the national institutes of health Stroke Scale scores were 9 and 18, respectively. both patients underwent intravenous thrombolysis followed by endovascular thrombectomy, and successful reperfusion was achieved, as shown by a modified Thrombolysis in cerebral infarction Tici (mTici) scale score of 2c at 1.5 hours from symptom onset in the patient aged 50−59 and by a mTici scale score of 3 at 4 hours from symptom onset in the patient aged 80−89. dynamic contrast-enhanced magnetic resonance (mR) angiography (dce-mRA in [A and h]) at admission showed steno-occlusion of the left middle cerebral arteries (mcAs), and apparent diffusion coefficient (Adc) images (b and i) at admission showed similar size baseline lesions in the left mcA territories. The collateral maps derived from dce-mRA (c-f and j-m) of both patients show very poor collateral perfusion status (mAc 0 : collateral perfusion delay/defect more than one-half of the mcA territory in the late venous phase regardless of collateral perfusion status at previous phases) [7,21]. fluid-attenuation inversion recovery magnetic resonance image (flAiR) on day 1 in the patient in his 50s (g) shows a decreased extent of the baseline lesion. The 90-day mRS score of this patient was 2. computed tomography (cT) image on day 1 in the patient in his 80s shows progression of malignant edema (n). The 90-day mRS score of this patient was 5.
The collateral perfusion grading system was defined by using the MR acute ischemic stroke collateral (MAC) scores as follows : 5 (excellent), no or collateral perfusion delay in the MCA territory in the capillary phase; 4 (good), collateral perfusion delay ≤1/2 of MCA territory in the capillary phase and no or small delay in the early venous phase; 3 (intermediate to good), 1) collateral perfusion delay ≤1/2 of MCA territory in the capillary and early venous phases, 2) collateral perfusion delay >1/2 of MCA territory in the capillary phase and no or small delay of MCA territory in the early venous phase; 2 (intermediate to poor), collateral perfusion delay >1/2 of MCA territory in the capillary phase and ≤1/2 of MCA territory in the early venous phase; 1 (poor), collateral perfusion delay >1/2 of MCA territory in the early venous phase and ≤1/2 of MCA territory in the late venous phase; and 0 (very poor), collateral perfusion delay >1/2 of MCA territory until the late venous phase [7]. Two experienced neurointerventionists who were blinded to all clinical and other imaging data independently graded the MAC scores of the MR angiography collateral maps. Two raters determined the final scores by consensus. An experienced neurologist measured the infarct volume (in milliliters) using Medical Image Processing, Analysis, and Visualization (MIPAV) version 7.1.1 (National Institute of Health, Bethesda, MD, USA).
Statistical analysis
The participant characteristics are expressed as the mean± standard deviation, median (interquatile range, IQR), or number of participants (%). Differences in the distribution of patient characteristics among collateral perfusion grades were assessed using the chi-square test, Fisher’s exact test, analysis of variance and the Kruskal-Wallis test, as appropriate. The interrater agreement for collateral perfusion grading was tested using Cohen’s kappa coefficient. Multiple logistic regression analyses were conducted to identify independent predictors of unfavorable functional outcomes in all participants and participants who achieved successful reperfusion. Variables are presented in Table 1. We included predictors in multivariable models according to clinical significance in previous studies in multivariable models [7,19,20,24]. The results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). To examine the linearity of the relationship between collateral perfusion grades and functional outcomes, the likelihood ratio test for trends was used. Subgroup analyses using multiple logistic regression were conducted to identify independent predictors of favorable functional outcomes in participants with a collateral perfusion grade of MAC 0. The age threshold was analyzed for the prediction of favorable functional outcomes in participants with a collateral perfusion grade of MAC 0 using receiver operating characteristic analysis with calculation of the area under the curve and subsequent determination of Youden’s J index to establish cutoff points. Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). A 2-sided p-value of <0.05 was considered significant.
RESULTS
Participant characteristics and collateral perfusion grading
From January 2016 to March 2021, 214 of 633 participants were included in this study (Fig. 2). The mean age was 70±12 years (range, 33–94), and 60% were men (129 men and 85 women). The median baseline NIHSS score was 12 (IQR, 7–15), and the median baseline DWI lesion volume was 14.2 mL (IQR, 5.0–39.8). The median onset-to-door time was 44 minutes (IQR, 28–115), and the median onset-to-reperfusion time was 220 minutes (IQR, 170–315). A total of 116 participants (54.2%) underwent EVT, and 98 participants (45.8%) underwent intravenous thrombolysis followed by EVT. A total of 184 participants (86%) achieved successful reperfusion.

flowchart shows study selection according to the exclusion criteria. dASAn : database of Acute ischemic Stroke Analysis network, eVT : endovascular thrombectomy, mRS : modified Rankin scale.
The interrater correlation between the two observers showed almost perfect strength of agreement for collateral perfusion grading of the collateral map (weighted κ, 0.96; 95% CI, 0.93 to 0.98). The baseline clinical characteristics for each collateral perfusion grade are presented in Table 1. Lower baseline NIHSS scores and smaller baseline DWI lesion volumes were associated with higher collateral perfusion grades (all p<0.001). Other characteristics did not differ among participants in different collateral perfusion grades.
Predictors of unfavorable functional outcomes after EVT
The results of the univariable and multivariable logistic regression analyses performed to identify independent predictors of unfavorable functional outcomes in all participants are presented in Table 2. In the multivariable analysis, older age (OR, 2.40; 95% CI, 1.56 to 3.67; p<0.001), higher baseline NIHSS scores (OR, 1.12; 95% CI, 1.04 to 1.21; p=0.004), very poor collateral perfusion grade (OR, 35.09; 95% CI, 3.50 to 351.33; p=0.002), longer door-to-groin puncture time (OR, 1.08; 95% CI, 1.02 to 1.14; p=0.009), and failed reperfusion (OR, 3.73; 95% CI, 1.30 to 10.76; p=0.015) were independently associated with unfavorable functional outcomes. A linear negative association was found between collateral perfusion grades and functional outcomes (p=0.005 by linear trend test). In 184 participants (mean age, 70±12 years; 118 men) who achieved successful reperfusion, the multivariable analysis showed that older age (OR, 2.30; 95% CI, 1.44 to 3.67; p<0.001), higher baseline NIHSS scores (OR, 1.12; 95% CI, 1.03 to 1.22; p=0.006), very poor collateral perfusion grade (OR, 4.96; 95% CI, 1.42 to 17.37; p=0.012), and longer door-to-reperfusion time (OR, 1.09; 95% CI, 1.03 to 1.15; p=0.003) were independently associated with unfavorable functional outcomes (Table 3).
Despite significant results in the univariable analysis and clinical significance, we could not include several time variables in multivariable analyses because of the lack of those variables in some participants with unclear onset or failed reperfusion.
Predictors of favorable functional outcomes in participants with very poor collateral perfusion status (MAC 0)
We conducted subgroup analysis to identify independent predictors of favorable functional outcomes in 38 participants with MAC 0 collateral perfusion grade. This grade was revealed as the major predictor with the highest OR of unfavorable functional outcomes after EVT. The mean age was 73±11 years (range, 49–91), and 71.1% were men (27 men and 11 women). Of 38 participants, 30 participants showed unfavorable outcomes (78.9%). In univariable and multivariable analyses, younger age (mean, 65±10 years; range, 51–83 years in favorable outcomes vs. mean, 75±10 years; range, 49–91 years in unfavorable outcomes) (OR, 0.29; 95% CI, 0.10 to 0.85; p=0.025) and the presence of previous ischemic heart disease (four participants [50%] in favorable outcomes vs. two participants [6.7%] in unfavorable outcomes) (OR, 23.63; 95% CI, 2.07 to 269.53; p=0.011) were associated with favorable functional outcomes. The age threshold for favorable functional outcomes after EVT in participants with MAC 0 collateral perfusion grade was ≤73 years of age with 87.5% sensitivity (95% CI, 47.3 to 99.7) and 63.3% specificity (95% CI, 43.9 to 80.1) (Fig. 3).
DISCUSSION
Our study demonstrated that older age, higher baseline NIHSS score, very poor collateral perfusion grade (MAC 0), longer door-to-groin puncture time, and failed reperfusion were significantly associated with unfavorable outcomes after EVT in acute ischemic stroke due to occlusion of the internal carotid artery and/or MCA (M1 or M2 segments) within 8 hours of symptom onset. In patients who achieved successful reperfusion, older age, higher baseline NIHSS score, very poor collateral perfusion grade, and longer door-to-reperfusion time were significantly associated with unfavorable outcomes. In patients with very poor collateral perfusion status, younger age and the presence of previous ischemic heart disease were significantly associated with favorable functional outcomes.
This study suggested a specific collateral perfusion grade beyond the classic guideline of baseline lesion size to identify ineligible patients for EVT. Age, baseline NIHSS scores, and baseline lesion size are the main factors in current EVT guidelines for patients with acute anterior circulation ischemic stroke [20]. Our results showing that age and baseline NIHSS score are the main prognostic factors are consistent with the results of previous studies [6,10,25]. However, baseline DWI lesion volume was not associated with unfavorable functional outcomes. Baseline lesion size on CT, CT perfusion or DWI has been a major decision factor in EVT. The underlying assumption is that patients with a large baseline lesion size have less salvageable tissue and are thus unlikely to benefit from EVT. However, previous studies have shown positive results with EVT in large baseline lesions [2,22,28]. Current imaging methods have the limitation of uncertainty about the discrimination of irreversibly injured brains and injured but salvageable brains [5,8], which makes it possible to exclude patients who are still likely to benefit from EVT despite large baseline lesions. Tissue fate mainly depends on collateral circulation. The impact on functional outcomes of collateral circulation can be greater than that of the baseline lesion size, as shown in previous studies [7,9,13,27]. In terms of futile EVT, Pan et al. [19] used digital subtraction angiography for collateral estimation and reported that old age, high baseline NIHSS, and poor collateral circulation are risk factors for futile EVT in acute ischemic stroke patients treated with EVT. Baseline lesion size was not associated with functional outcomes in the study. Those results were consistent with ours. Among the six collateral perfusion grades of the collateral map, MAC 0 reflects a severely hypoperfused region in more than one-half of the area of the MCA territory where collateral perfusion delay/defects persist until the late venous phase of the patient’s circulation; a grade of MAC 0 showed the highest OR among the predictors of unfavorable functional outcomes in all participants and participants who achieved successful reperfusion in the current study.
In patients with very poor collateral perfusion status, the age threshold of favorable functional outcomes was ≤73 years. A meta-analysis showed that patients aged ≥80 years had worse functional outcomes (p<0.001) and higher rates of mortality (p<0.001) after EVT [29]. Alawieh et al. [1] also reported that age ≥80 years was independently associated with increased mortality, poor outcome, and a higher rate of postprocedural hemorrhage. With knowledge of the results from these studies and our analyses, we can make different treatment decisions in octogenarian patients with very poor collateral perfusion status who are unlikely to benefit from EVT. Further randomized controlled studies are necessary to develop specific guidelines for EVT eligibility in elderly patients with very poor collateral status.
In the current study, the presence of previous ischemic heart disease was associated with favorable functional outcomes in participants with very poor collateral perfusion status. Only six patients had previous ischemic heart disease. The impact on functional outcomes of previous ischemic heart disease in this group is not conclusive until further studies are conducted in a large population.
The limitation of this study is that the study population was too small to specify the impact of onset-to-reperfusion time on futile EVT in different collateral perfusion grades. In a multicenter retrospective study of 554 patients with EVT (of whom 86 had a poor collateral grade estimated using single phase CT angiography), not a single patient with poor collaterals and an onset-to-reperfusion time >174 minutes had favorable functional outcomes (mRS score 0–2 at 3 months). They showed that earlier successful reperfusion was strongly associated with favorable functional outcomes in the poor collateral group; however, this association was weak in the good collateral group, which suggests that the onset-to-reperfusion time window for favorable functional outcomes can be adjusted according to collateral status [6]. Despite significant results of univariable analysis for onset-to-reperfusion time, we could not include it in multivariable analysis due to lack of the variable in some patients with unclear onset. However, the door-to-reperfusion time was associated with functional outcomes in patients who achieved successful reperfusion by EVT. Collateral circulation determines the pace of infarct progression. Earlier reperfusion may be linked with poorer collateral status for achieving favorable functional outcomes. Further studies in a large population are necessary to identify the optimal time window according to collateral perfusion status for better outcomes of EVT. It will help to establish more specific guidelines to reduce futile EVT. Another limitation is that the collateral map can only be generated with additional software that is not currently commercially available. We expect that collateral maps can be generated using the dynamic information from computer tomography perfusion. It is necessary to establish a universally applicable foundation through ongoing efforts for the development and commercialization of a sophisticated collateral perfusion imaging.
CONCLUSION
Precise assessment of the collateral perfusion status is mandatory for determining eligibility of patients for EVT. This study demonstrates that EVT in elderly patients with very poor collateral perfusion status can be futile. The assessment of collateral perfusion status using the collateral map can predict futile EVT, which may help select ineligible patients for EVT, thereby potentially reducing the rate of futile EVT.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author contributions
Conceptualization : HJ Ki, YSJ, HJ Kim, HGR, JSL; Data curation : HJ Ki, YSJ, HJ Kim, HGR, TJL, JJP, SBL, HJL; Formal analysis : JSL; Funding acquisition : HJ Ki, HJ Kim; Methodology : HGR, JTK; Project administration : HJ Kim, HGR; Visualization : HJ Ki, HJ Kim; Writing - original draft : YSJ, HJ Kim, HGR, TJL, JJP, SBL, HJL, JTK, JSL, HJ Ki; Writing - review & editing : HJ Ki, YSJ, HJ Kim, HGR
Data sharing
None
Preprint
None
Acknowledgements
This study was supported by the National Research Foundation of Korea as subject number “2020R1F1A1071619”, “RS-2023-00252980”, the Ministry of Education of Korea as subject number “RS-2023-00248375”, and the Ministry of Health and Welfare of Korea as subject number “RS-2023-00266130”.