De Novo Vertebral Artery Dissecting Aneurysm after Parent Artery Occlusion of the Contralateral Vertebral Artery
Article information
Abstract
After treatment of unilateral vertebral artery dissecting aneurysm (VADA), de novo VADA rarely occurs on the contralateral side. In this article, we report a case of subarachnoid hemorrhage (SAH) due to de novo VADA in the contralateral vertebral artery (VA) 3 years after parent artery occlusion of unilateral VADA, with a review of the literature. A 47-year-old woman was admitted to our hospital complaining of headache and impaired consciousness. Head computed tomography showed SAH, and three-dimensional computed tomography angiography showed a fusiform aneurysm in the left VA. We performed an emergency parent artery occlusion. Three years and 3 months after the initial treatment, the patient presented to our hospital with complaints of headache and neck pain. Magnetic resonance imaging revealed SAH, and magnetic resonance angiography revealed de novo VADA in the right VA. We performed a stent-assisted coil embolization. The patient had a good postoperative course and was discharged with a modified Rankin scale score of 0. Long-term follow-up is necessary in patients with VADA because contralateral de novo VADA can develop even several years after the initial treatment.
INTRODUCTION
Ruptured vertebral artery dissecting aneurysms (VADAs) have a high risk of rebleeding in the acute phase; rebleeding causes poor outcomes, with a mortality rate as high as 45% [5,14]. We must prevent rebleeding at an early stage. The most effective treatment for preventing re-rupture is parent artery occlusion or internal trapping [13]. After the initial treatment with unilateral VADA, de novo VADA rarely occurs on the contralateral side.
We report a case of de novo VADA in the contralateral vertebral artery (VA) 3 years after parent artery occlusion for unilateral VADA, resulting in subarachnoid hemorrhage (SAH), along with a review of the literature.
CASE Report
A 47-year-old woman was admitted to our institution complaining of headache, nausea, and disturbance of consciousness. Head computed tomography (CT) revealed SAH in the posterior cranial fossa (Fig. 1A). Three-dimensional CT angiography revealed a dissecting aneurysm in the left intracranial VA (Fig. 1B). Digital subtraction angiography (DSA) reveled a left VADA that bifurcated into the posterior inferior cerebellar artery (PICA) (Fig. 1C). We determined to be the source of the bleeding. We performed an emergency parent artery occlusion using eight detachable coils (Fig. 1D). The patient was treated to preserve left PICA patency (Fig. 1E). Magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) showed a high-intensity signal in the bilateral cerebellar hemispheres (Fig. 1F). The patient underwent postoperative rehabilitation and was discharged home with a modified Rankin scale (mRS) score of 1, although mild abducens nerve palsy persisted. Thereafter, we continued follow-up MRI and DSA, and the imaging findings remained unchanged until 34 months after the initial treatment (Fig. 2).

A : Brain computed tomography (CT) scan shows diffuse subarachnoid hemorrhage in the prepontine cistern. B : Three-dimensional CT angiography demonstrates a fusiform aneurysm of the left vertebral artery (VA). C : Left vertebral angiography shows a fusiform dilatation of the left VA. D : Parent artery occlusion for left vertebral artery dissecting aneurysm is performed. E : Right vertebral angiography shows the left posterior inferior cerebellar artery patency after parent artery occlusion. F : Magnetic resonance imaging shows diffusion-weighted imaging high intensity area in bilateral cerebellar hemisphere.
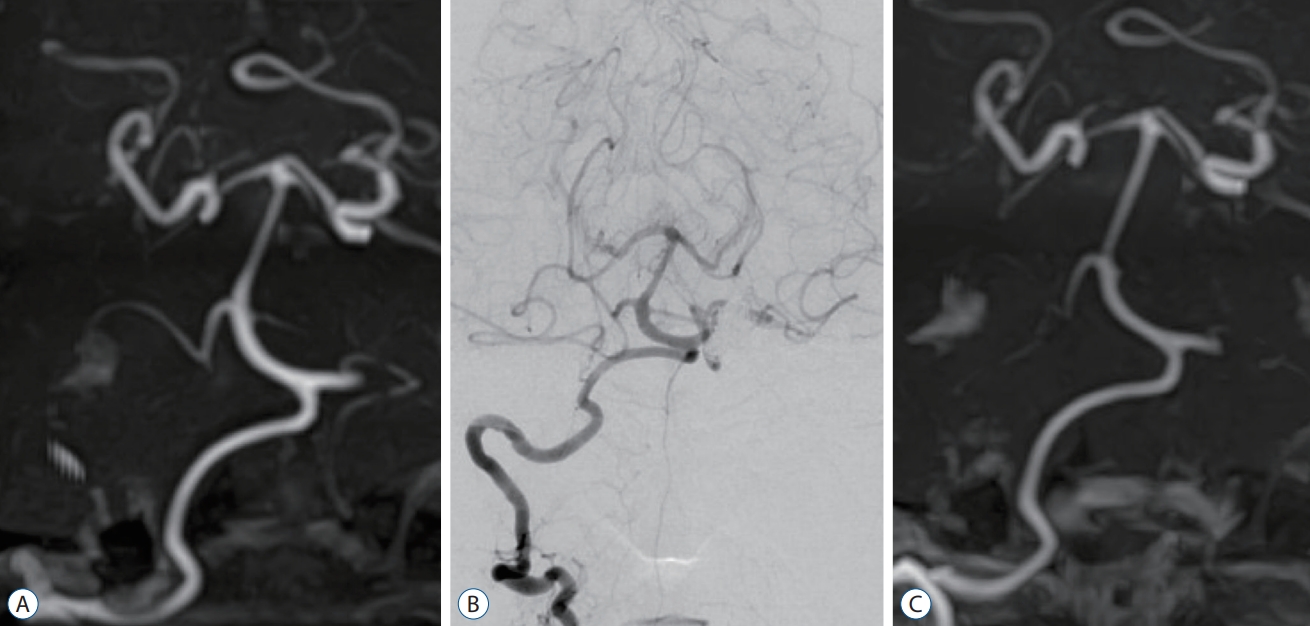
A : Magnetic resonance angiography 2 months postoperatively shows no dilatation in the right vertebral artery (VA). B : Follow-up digital subtraction angiography performed 16 months postoperatively shows no dilatation of the right VA. C : Magnetic resonance angiography 3 years after the procedure shows no abnormal findings in the right VA.
Five months after the last MRI examination, the patient developed severe posterior neck pain and was admitted to our hospital as an emergency department. MRI revealed SAH, and magnetic resonance angiography (MRA) revealed de novo VADA in the right VA (V4 segment) (Fig. 3A and B). We decided to perform stent-assisted coil embolization (SAC) after administering a loading dose of aspirin (300 mg) and clopidogrel (300 mg) 2 hours before starting endovascular treatment. We guided a Headway 21 (Terumo., Tokyo, Japan) to the VA union and then placed an Excelsior SL-10 microcatheter (Stryker, Kalamazoo, MI, USA) for aneurysmal dilatation. An low-profile visualized intraluminal supprt blue 3.5×23-mm stent (MicroVention, Tustin, CA, USA) was placed through the proximal anterior spinal artery bifurcation, and seven detachable platinum coils were inserted through the jailed SL-10 with flow preservation of the Rt VA (Fig. 3C-E). Postoperative DWI revealed no apparent embolism, and the patient had no neurological deficits (Fig. 3F). Follow-up DSA on day 28 showed no evidence of VADA recurrence. This patient was discharged with an mRS score of 0 after rehabilitation. The patient was scheduled to continue dual antiplatelet therapy (DAPT) for the first 6 months after the second treatment.

A : Fluid attenuated inversion recovery image on admission shows subarachnoid hemorrhage in the prepontine cistern. B : Magnetic resonance angiography reveals dilatation of the right vertebral artery (VA), indicating a VA dissecting aneurysm. C : Right vertebral angiography reveals de novo vertebral artery dissecting aneurysm (VADA). D : After stent-assisted coil embolization of the right VADA, the right vertebral angiography demonstrates occlusion of the aneurysm dilatation. E : Coil embolization with low-profile visualized intraluminal supprt blue stent (MicroVention, Tustin, CA, USA) is performed. F : Diffusion-weighted imaging reveals no high-intensity area.
DISCUSSION
The rebleeding rate of ruptured VADA is high; therefore, therapeutic intervention should be provided as early as possible [5,14]. The location of the PICA bifurcation and dissecting aneurysm and the degree of development of the contralateral VA are key factors in deciding the treatment strategy for endovascular treatment of ruptured VADA. In ruptured cases, the efficacy of parent artery occlusion or internal trapping has been established, and its greatest advantage is the ability to achieve hemostasis [13]. However, there is a risk of infarction in the perforator territory depending on the location of aneurysm and the distance of the embolization [3].
In cases of VADA with occlusion or hypoplasia of the contralateral VA and PICA-involved type, a SAC is now being performed in the acute stage of SAH [2,8,9]. While this treatment has the advantage of being less invasive and preserving blood flow of the parent artery, risk of recurrence remains a major problem. Cho et al. [1] reported a high recurrence rate with SAC for VADA of the PICA-involved type. Therefore, Kim et al. [10] proposed that a staged and combined strategy could be another treatment option for treating VADA involving the PICA. They reported four cases of embolization of the rupture point in the acute stage and then occlusion of the proximal VA and PICA origin after occipital artery (OA)-PICA bypass in the chronic stage, resulting in favorable outcomes.
Risk factors for VA dissection have been noted to include trauma, migraine, pregnancy, connective tissue disease (e.g., Marfan syndrome), and hypertension [4,17]. In the present case, the only risk factor was hypertension. In this case, a pre-PICA-type VADA was observed on the left side of the VA at the initial treatment. Because the contralateral VA was also well developed, we performed a parent artery occlusion. Five months before the second treatment, MRA showed no imaging changes. However, 38 months after the first treatment, the patient developed SAH because of de novo VADA on the contralateral side. Since this patient had already received parent artery occlusion for left VA, right VA trapping and OA-anterior inferior cerebral artery (AICA) bypass was considered as surgical treatment for this case. While this strategy could certainly prevent re-rupture, the risk of ischemic complications remains a major problem. Because of the loss of anterograde blood flow through the right VA, collateral blood flow from the OA-AICA bypass and posterior communication artery alone could not provide sufficient perfusion to the posterior cranial fossa. This case was a non-PICA type VADA, and we could not identify branched vessel or perforating artery from the dissected portion on angiography. SAC was determined to be the preferred treatment to prevent rebleeding.
SAC in acute SAH may be associated with hemorrhagic and thrombotic complications due to the administration of DAPT. Ryu et al. [18] reported a systematic review of the timing of loading DAPT for coil embolization with a stent in the acute phase of rupture. The analysis reported that administering a loading dose of DAPT for at least 2 hours before the start of treatment resulted in fewer ischemic events and no increase in hemorrhagic events compared with DAPT loading immediately after the start of treatment. In the present case, DAPT was administered at a loading dose 2 hours before the start of treatment, and no apparent perioperative complications were observed.
To the best of our knowledge, seven cases have been reported, including contralateral de novo VADA after surgical or endovascular occlusion of VADA or spontaneous occlusion of VADA. The previously reported cases are summarized in Table 1 [6-8,11,12,15]. All of the cases were reported from Japan, and the primary side was the left side in six cases. Including this case, six patients had SAH and underwent trapping or parent artery occlusion, and two patients had cerebral infarction and underwent conservative treatment. The time between the initial dissection and occurrence of a contralateral de novo aneurysm varies from patient to patient. In the present case, the period between the first dissection and the occurrence of de novo VADA was 38 months, which is the longest time reported among the reported.
The mechanism of contralateral de novo VADA remains unclear. There have been two reported cases in which VA trapping resulted in an increase in the diameter of the contralateral VA [6,19]. One possible cause is hemodynamic stress on the contralateral side due to occlusion of the unilateral VA. On the other hand, a case of de novo VADA on the contralateral side after treatment with unilateral VADA with a flow-diveter stent has been reported [20]. This suggests a mechanism other than hemodynamic stress. Ro et al. [16] investigated the pathology of 58 cases of VADA in SAH and reported that 17.2% (10/58) had dissection in the bilateral VA. It is suggested that there could be a dissecting lesion that was undetectable on imaging, even if unilaterally VADA was present. Long-term follow-up is necessary, considering the possibility that de novo VADA could also develop on the contralateral side, as in the present case.
CONCLUSION
We report a case of de novo VADA in the contralateral VA that developed SAH 3 years after parent artery occlusion for unilateral VADA. When treating patients with VADA, long-term follow-up is needed because contralateral de novo VADA can occur several years after the initial occurrence of VADA.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
Informed consent was obtained from the patient included in this study.
Author contributions
Conceptualization : YT, SM; Data curation : YT, SM; Formal analysis : YT; Methodology : YT; Project administration : YT; Visualization : YT, YK; Writing - original draft : YT; Writing - review & editing : YK, MW
Data sharing
None
Preprint
None
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.