Morbidity and Mortality Trends in Preterm Infants of <32 Weeks Gestational Age with Severe Intraventricular Hemorrhage : A 14-Year Single-Center Retrospective Study
Article information
Abstract
Objective
Owing to advances in critical care treatment, the overall survival rate of preterm infants born at a gestational age (GA) <32 weeks has consistently improved. However, the incidence of severe intraventricular hemorrhage (IVH) has persisted, and there are few reports on in-hospital morbidity and mortality. Therefore, the aim of the present study was to investigate trends surrounding in-hospital morbidity and mortality of preterm infants with severe IVH over a 14-year period.
Methods
This single-center retrospective study included 620 infants born at a GA <32 weeks, admitted between January 2007 and December 2020. After applying exclusion criteria, 596 patients were included in this study. Infants were grouped based on the most severe IVH grade documented on brain ultrasonography during their admission, with grades 3 and 4 defined as severe. We compared in-hospital mortality and clinical outcomes of preterm infants with severe IVH for two time periods : 2007–2013 (phase I) and 2014–2020 (phase II). Baseline characteristics of infants who died and survived during hospitalization were analyzed.
Results
A total of 54 infants (9.0%) were diagnosed with severe IVH over a 14-year period; overall in-hospital mortality rate was 29.6%. Late in-hospital mortality rate (>7 days after birth) for infants with severe IVH significantly improved over time, decreasing from 39.1% in phase I to 14.3% in phase II (p=0.043). A history of hypotension treated with vasoactive medication within 1 week after birth (adjusted odds ratio, 7.39; p=0.025) was found to be an independent risk factor for mortality. When comparing major morbidities of surviving infants, those in phase II were significantly more likely to have undergone surgery for necrotizing enterocolitis (NEC) (29.2% vs. 0.0%; p=0.027). Additionally, rates of late-onset sepsis (45.8% vs. 14.3%; p=0.049) and central nervous system infection (25.0% vs. 0.0%; p=0.049) were significantly higher in phase II survivors than in phase I survivors.
Conclusion
In-hospital mortality in preterm infants with severe IVH decreased over the last decade, whereas major neonatal morbidities increased, particularly surgical NEC and sepsis. This study suggests the importance of multidisciplinary specialized medical and surgical neonatal intensive care in preterm infants with severe IVH.
INTRODUCTION
Intraventricular hemorrhage (IVH) is a major complication of premature birth [25]. The incidence of IVH is higher in infants born at an early gestational age (GA) and is especially high in infants born at a GA <32 weeks [26]. In a recent Korean national report on IVH in very low birth weight (VLBW) infants, the incidence rate of severe IVH (defined as grades 3 and 4 by the Papile grading system) was 10.3%, which, despite the overall decline in IVH, persisted because of the consistently increasing survival rate [1].
IVH grading is traditionally based on the Papile grading system and ranges from mildest (grade I) to most severe (grade IV) [20]. In mild IVH (grades I and II), long-term neurodevelopmental outcomes of infants born at an extremely early GA are unknown, but severe IVH (grades III and IV) is highly associated with cognitive disabilities such as cerebral palsy and intellectual deficits [22].
IVH typically occurs within the first 3 days after birth and can progress rapidly in early life (within 1 week of birth) [9,27]. Until discharge, treatment for this patient population is complex due to the presence of multisystem co-morbidities requiring multiple subspecialists. Additionally, patient prognosis is affected by many factors including GA, birth weight, post-hemorrhagic hydrocephalus (PHH), and complications typically associated with prematurity [5]. Advances in neonatal care decreased incidence of neonatal morbidities in preterm infants; however, significant morbidities remain a concern in infants with severe IVH. Currently, there are limited studies on in-hospital mortality and co-morbidities in preterm infants with severe IVH.
The present study aimed to investigate trends in in-hospital morbidity and mortality of preterm infants with severe IVH at a single center over a 14-year period. We also explored risk factors contributing to mortality in preterm infants with severe IVH.
MATERIALS AND METHODS
The protocol for the present study was approved by the Institutional Review Board of Korea University Ansan Hospital (2021AS0281).
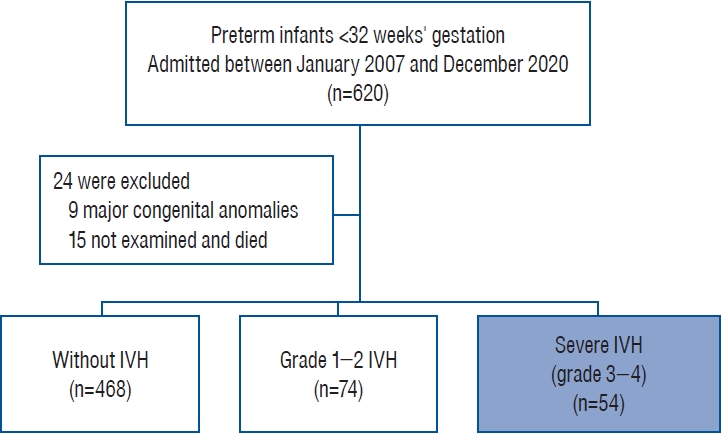
The present study was a single-center retrospective study using data from preterm infants born at <32 weeks GA and admitted to the neonatal intensive care unit (NICU) of the Korea University Ansan Hospital from January 2007 to December 2020. Infants with fetal hydrops (n=3), biliary atresia (n=1), major heart defects (n=3), or brain anomalies (n=2) were excluded as confounding risk factors for mortality. Additionally, 15 infants were excluded because there were no records of brain ultrasonography (BUS) results. After applying the exclusion criteria, 596 patients were included in this study.
BUS
Ultrasound examinations were performed by a pediatric radiologist. The Papile grading system was used to assess and grade IVH as follows : grade 1, hemorrhage restricted to the periventricular germinal matrix regions or germinal matrix; grade 2, IVH without ventricular dilatation; grade 3, hemorrhage extending into dilated ventricles; and grade 4, hemorrhage within the ventricular system and parenchyma. Infants were grouped according to the highest grade documented on the BUS performed during hospitalization in the NICU; those categorized as grade 3 or 4 were defined as severe IVH.
Clinical data
Birth and neonatal clinical characteristics were documented, including GA and birth weight at delivery, sex, mode of delivery, 1-minute Apgar score, 5-minute Apgar score, need for resuscitation in the delivery room, use of antenatal steroids, use of vasoactive medications, red blood cell or platelet transfusions, and premature membrane rupture. Laboratory results included the initial capillary blood gas from arterial blood samples and the initial hemoglobin and platelet levels from venous blood samples, measured after birth. The following clinical outcomes were also documented : surfactant use in infants with respiratory distress syndrome, patent ductus arteriosus treatment with intravenous ibuprofen or surgical ligation, necrotizing enterocolitis (NEC) with radiologic evidence of pneumatosis intestinalis and its associated treatment, bronchopulmonary dysplasia (BPD; defined as the requirement for supplemental oxygen with a fraction of inspired oxygen >0.21 for 28 days of life), the use of steroids to treat BPD, retinopathy of prematurity and its associated treatment, early onset sepsis (<72 hours after birth) proven by blood cultures, late-onset sepsis, central nervous system (CNS) infection, periventricular leukomalacia diagnosed by BUS or brain magnetic resonance imaging, PHH with external ventricular drainage (EVD), and/or a shunt operation prior to discharge. Mild BPD was defined as breathing room air, moderate BPD was defined as having an FiO2 <0.30, and severe BPD was defined as having an FiO2 ≥0.30 or positive pressure ventilation at 36 weeks post-menstrual age.
We compared the incidence rate of in-hospital mortality in preterm infants with severe IVH during two time periods : 2007–2013 (phase I) and 2014–2020 (phase II). Baseline characteristics of infants who died during hospitalization and those who survived NICU hospitalization were analyzed, and risk factors for mortality were identified. We then compared the clinical outcomes of the survivors from each time period.
Statistical analysis
Continuous variables were analyzed using either the t-test or the Mann-Whitney U test for normal or skewed distributions, respectively. Proportions were evaluated using chi-square and Fisher’s exact tests, and statistical significance was set at p<0.05. Univariate and multivariate logistic regression analyses were performed to identify risk factors for in-hospital mortality; results are reported as odds ratios (ORs) and 95% confidence intervals (CIs). Data are presented as mean±standard deviation, median and range, or rate.
RESULTS
For preterm infants born at <32 weeks GA during phases I and II, there was no statistically significant difference in GA (29.2±2.2 weeks vs. 29.0±2.3 weeks, respectively; p=0.327) or birth weight (1304±394 g vs. 1286±383 g, respectively; p=0.574). The overall incidence of IVH was 21.5% (128 of 596 infants), while severe IVH occurred in 9.0% (54 of 596) of infants over the 14-year period (Fig. 1). The incidence rate of severe IVH changed from 8.1% (24 of 297) in phase I to 10.0% (30 of 299) in phase II, although this difference was not statistically significant (p=0.476). The overall in-hospital mortality rate of infants with severe IVH during the 14-year period was 29.6% (16 of 54). The overall mortality rate decreased from 41.7% (10 of 24) in phase I to 20.0% (6 of 30) in phase II (p=0.083), despite earlier GA of infants included in phase II (27.4±2.1 weeks vs. 26.3±2.3 weeks, respectively; p=0.051) (Table 1). Notably, the late (>7 days after birth) in-hospital mortality rate significantly improved from 39.1% in phase I to 14.3% in phase II (p=0.043) (Fig. 2), and the mortality rate of grade 4 IVH infants was higher than that of grade 3 infants (37.8% and 11.8%, respectively).
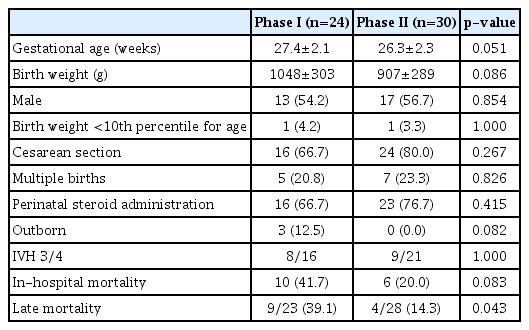
Clinical characteristics and mortality of preterm infants with severe IVH during two time periods, 2007–2013 (phase I) and 2014–2020 (phase II)
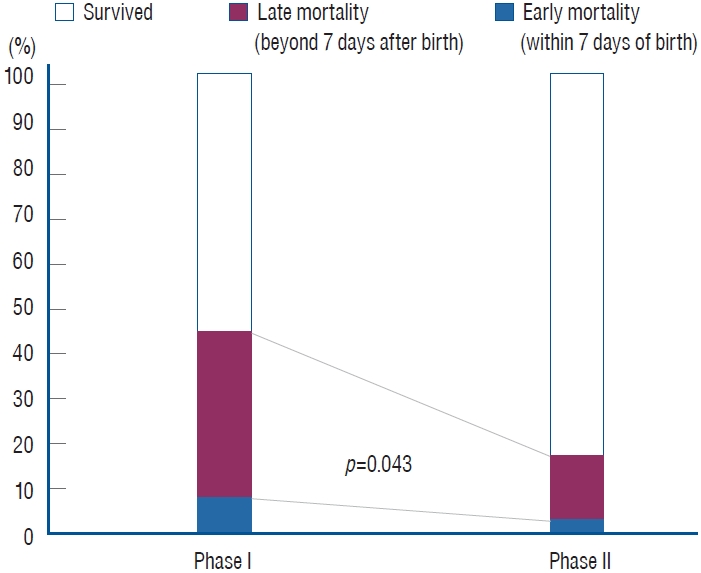
Trend of in-hospital mortality in infants with severe intraventricular hemorrhage over a 14-year period; late in-hospital mortality rates significantly improved from 39.1% in phase I to 14.3% in phase II (p=0.043).
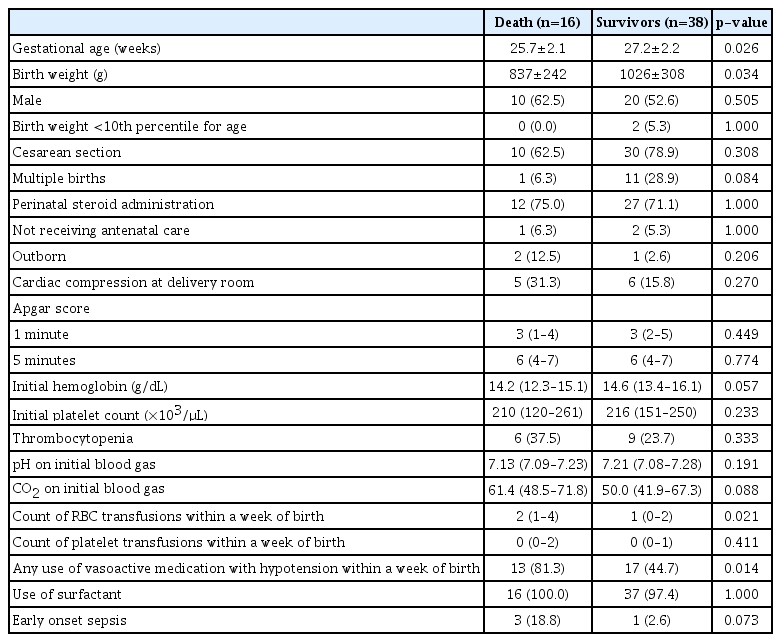
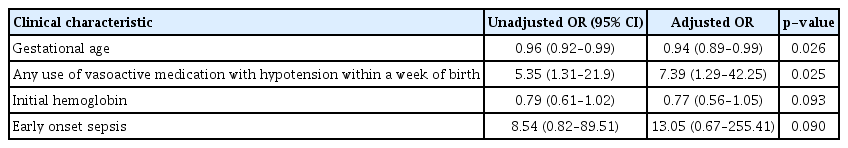
In-hospital death was significantly associated with early GA (p=0.026), lower birth weight (p=0.034), number of red blood cell transfusions (p=0.021), and a history of hypotension with the use of vasoactive medications within a week of birth (p=0.014) (Table 2). In the multivariate logistic regression analysis, after adjusting for variables, hypotension with the use of inotropes within a week (adjusted OR, 7.39; 95% CI, 1.29–42.25; p=0.025) was found to be an independent risk factor for mortality, while a later GA (adjusted OR, 0.94; 95% CI, 0.89–0.99; p=0.026) was found to be a protective factor (Table 3).
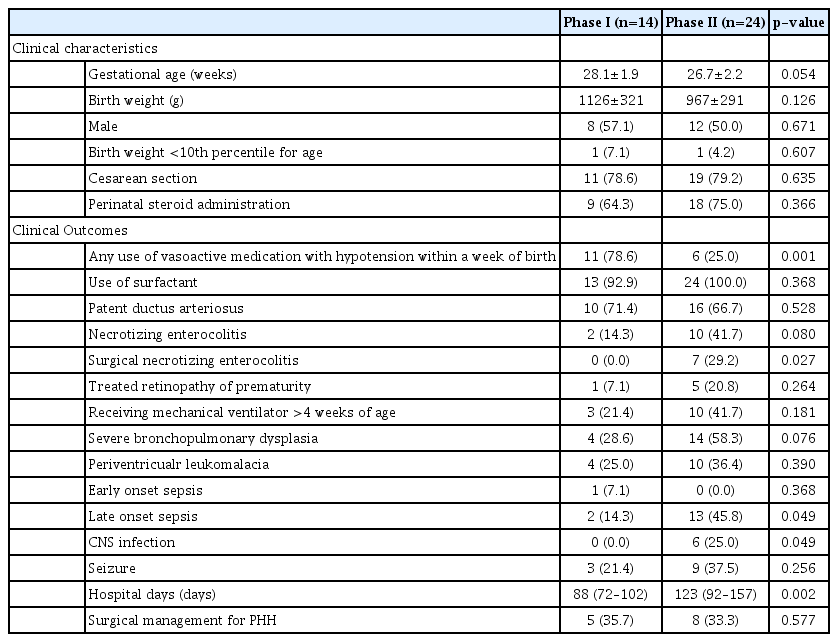
Table 4 shows a comparison of clinical characteristics and outcomes of surviving infants for each phase of the study. Survivors in phase II were younger than those in phase I (28.1 ±1.9 weeks vs. 26.7±2.2 weeks, respectively; p=0.054), as well as smaller (1126±321 g vs. 967±291 g, respectively; p=0.126) without statistical significance. The use of inotropes within a week was less frequent in phase II than in phase I survivors (78.6% vs. 25.0%, respectively; p=0.001), while phase II survivors with severe IVH were more likely to undergo surgery for NEC than those in phase I (0.0% vs. 29.2%, respectively; p=0.027). The rate of late-onset sepsis was significantly higher in phase II survivors than in phase I survivors (14.3% vs. 45.8%, respectively; p=0.049), as was the case for CNS infections (0.0% vs. 25.0%, respectively; p=0.049). Median hospitalization duration was longer in the phase II group than in the phase I group (p=0.02), although there was no difference in the frequency of surgical treatment for PHH between phases I and II (35.7% vs. 33.3%, respectively; p=0.577). Except for one infant who underwent EVD in phase II, all cases of surgical treatment during hospitalization survived and were discharged.
DISCUSSION
In the present study, 21.5% of infants born at <32 weeks GA had IVH. The incidence rate of severe IVH was 9.0%, although the in-hospital mortality rate of infants with severe IVH was 29.6%. The Korean Neonatal Network reported a similar incidence rate, with severe IVH documented in 10.3% of VLBW infants [1,15]. Prior studies on mortality in infants with severe IVH reported rates of 30–60%, which is directly correlated with degree of severity and varies substantially between geographic regions [4,11,14,18]. At our institution, 87.5% of infants with severe IVH who died had grade 4 IVH, and mortality rates of infants with grade 3 and 4 IVH were 11.8% and 37.8%, respectively.
Mortality rate of infants with severe IVH decreased over a 14-year period, particularly the mortality rate after surviving for at least 1 week, which decreased by 21.7% in phase II (2013–2020) compared with that in phase I (2007–2013). Short- and long-term outcomes of VLBW infants in Korea continue improving [15]; however, previous studies investigating trends in morbidity and mortality for preterm infants did not report outcomes for infants with severe IVH separately. In the present study, although there was an overall trend of declining mortality, more recently, there was a significant increase in major neonatal morbidities in infants with severe IVH such as late-onset sepsis, NEC, and/or BPD. Late-onset sepsis and NEC in infants who would have otherwise died before reaching the vulnerable postnatal age, after which late-onset sepsis or NEC commonly occurs, are likely to be a result of improvements in NICU care, such as invasive procedures, central-line associated bloodstream infection prevention, and mechanical ventilation [16,21]. Patel et al. [21] analyzed NICU deaths among 22248 preterm infants with GAs of 22 0/7 to 28 6/7 weeks from 2000 through 2011 and found results similar to those of the present study, in that overall mortality decreased over time, particularly those related to pulmonary causes, immaturity, and infection, while deaths related to NEC increased.
NEC is the most common severe neonatal gastrointestinal complication and predominantly affects preterm infants [19]. Patients with NEC are known to be at a higher risk of developing IVH and/or progressing from mild IVH to more severe grades [13,17]. Recently, a multicenter prospective study showed that infants with IVH are more likely to develop NEC, particularly surgical NEC [7]. This result is consistent with that of the present study, which showed frequent coexistence of surgical NEC and IVH in survivors of severe IVH. NEC and IVH coexistence leads to a more complicated decision-making process because infants with severe IVH who require surgical treatment for PHH with gastrointestinal complications (NEC/ perforated bowel) require a ventriculoatrial rather than ventriculoperitoneal (VP) shunt [6]. If a VP shunt is utilized in infants with NEC, it can lead to gram-negative ventriculitis with serious effects in the brain [23]. Improved survival rate in VLBW infants with severe neonatal morbidities, particularly IVH and NEC, is likely to negatively impact long-term neurological outcomes [10,17]. Further investigation and guidelines regarding multidisciplinary management of comorbid NEC and IVH are needed.
Extremely preterm infants born before 28 weeks of gestation often have hypotension of various etiologies [12,24]. The association between hypotension and cerebral insult is well recognized, as hypotension may lead to impairment of cerebral autoregulation [2]. Some studies have identified the negative effects of standard antihypotensive treatments in preterm infants [3,8]. In the present study, significant hypotension, when treated with vasoactive medications within a week, was found to be an independent risk factor for mortality in infants with severe IVH. It should be noted that preterm infants with severe IVH have a history of significant hypotension within a week of birth.
The present study was limited by its retrospective design, small sample size, and utilization of only a single center. Large population-based multicenter studies investigating preterm infants with severe IVH are required to validate these results.
CONCLUSION
In conclusion, the results of the present study have shown that in-hospital mortality rates in preterm infants with severe IVH decreased over the 14 years evaluated, likely due to recent advances in neonatal intensive care, whereas the rates of some major neonatal morbidities, NEC, and late-onset sepsis increased. Hypotension, when treated with vasoactive medications within 1 week of birth, was found to be an independent risk factor for mortality in infants with severe IVH. These results support the need for specialized and focused medical and/or surgical neonatal care in preterm infants with severe IVH. Further research is needed on long-term prognosis and management of optimal neurodevelopment.
Notes
Conflicts of interest
Sang-Dae Kim has been editorial board of JKNS since May 2017. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : EKC, SDK; Data curation : EKC, BMC, BKJ; Formal analysis : EKC, SDK; Writing - original draft : EKC, SDK; Writing - review & editing : EKC, HK, BKJ, BMC, SDK
Data sharing
None
Preprint
None