Glossopharyngeal Neuralgia
Article information
Abstract
Glossopharyngeal neuralgia (GPN) is a rare disease that must be differentiated from trigeminal neuralgia. The purpose of this article is to provide a comprehensive review of anatomy, pathophysiology, diagnostic criteria, and several options of treatment for GPN. Lessons learned through our experience of treating GPN are presented in detail, as well as cases of misdiagnosis and diagnostic pitfalls. Microvascular decompression (MVD) should be primarily considered for medically intractable GPN. Techniques employed in MVD for GPN are categorized and described. Especially, we underscore the advantages of the ‘transposition’ technique where insulating material is positioned ‘off’ the root entry zone (REZ), instead of ‘on’ it. We believe this ‘off-the-REZ’ technique can fundamentally prevent recurrence, if applicable. In addition, Gamma Knife radiosurgery can be an alternative option when a patient is ineligible for MVD, though it is categorized as a destructive procedure.
INTRODUCTION
Glossopharyngeal neuralgia (GPN), albeit rare, poses a significant challenge to physicians. In 1910, the symptoms of GPN was first described in a case report on a patient who had been diagnosed with tic douloureux for 6 years; the authors suggested that the symptoms were attributed to a certain irritation of the ninth and twelfth cranial nerves [50]. The term GPN was first used by Harris to describe paroxysms of pain in the back of the tongue, throat, etc. in 1921 [12]. The incidence of GPN is roughly estimated as 1% of trigeminal neuralgia (TN) [38].
GPN is categorized as classical, secondary and idiopathic GPN in the classification of the International Headache Society [55]. In classical GPN, neurovascular compression on the glossopharyngeal nerve (GN) root is typically identified on magnetic resonance imaging or during MVD. The existence of an underlying disease accounting for the neuralgia constitutes the diagnosis of secondary GPN, whereas idiopathic GPN refers to a condition where neither an underlying disease nor neurovascular compression is demonstrated to cause the GPN symptoms. GPN, except for secondary one, affects only adults, with seemingly a predilection for the left side and the female gender, although no meta-analysis is available to verify the predilection [35].
The propriety of the term GPN has been questioned, for the syndrome may involve the sensory tributary of the vagus nerve (VN) as well, resulting in cardiovascular episodes, such as bradycardia, asystole or hypotension [7,36,50]. As we concur with Chen and Sindou [7], vago-glossopharyngeal neuralgia (VGPN) might be a better terminology than GPN. Our intraoperative findings of 30 GPN patients demonstrated that it was more common for a vessel or vessels to compress both the GN and VN rather than to compress the GN alone, due to their anatomical proximity [16].
Pathophysiology of GPN has been considered partially analogous to that of TN; thus, an assumption was made that treatment options for the latter could also be applied to the former. Accordingly, intracranial sectioning of the GN was performed by Singleton [43] and Dandy [8] in 1926 and 1927, respectively, and the concept of microvascular decompression (MVD) was first employed to this disease entity by Laha and Jannetta in 1977 [8,20,43]. MVD is the only therapeutic method that can offer a long-term cure for GPN. In this review, we will discuss and share our experience, especially focused on the application of transposition technique during MVD.
ANATOMY AND PATHOPHYSIOLOGY
Understanding surgical anatomy of the GN and pathophysiology of GPN is imperative to the establishment of treatment strategy. The GN is a mixed sensorimotor nerve which is small and lies deep within the neck, and is sometimes called ‘the neglected cranial nerve’, as it is often unnoticed in surgical dissections [32]. The GN projections exit the lateral aspect of the pons between the inferior olive and the inferior cerebellar peduncle. The somatic sensation of the GN is derived from the posterior two thirds of the tongue, middle ear, and pharynx, whereas the visceral sensation of it receives inputs from the carotid body. Parasympathetic fibers to the parotid gland also travel via the GN; they arise from the inferior salivatory nucleus of the pons and leave the GN as tympanic nerve, which then joins the lesser petrosal nerve. Motor fibers of the GN originate in the nucleus ambiguous of the medulla, and innervate stylopharyngeal muscle [32,47].
VN, a part of autonomic nervous system, comprises sensory and motor fibers, and interfaces with the parasympathetic control of the heart, lung and gastrointestinal tract. This nerve arises from the medulla oblongata and their rootlets exit lateral to olive between the olive and the inferior cerebellar peduncle [47]. The fibers from VN consist of branchial motor, visceral sensory, visceral motor, special and general sensory fibers. The brachial motor fibers of the VN are mainly involved in phonation and swallowing, by stimulating muscles in the pharynx, larynx, and the soft palate, as well as cardiac muscles. The visceral motor fibers stimulate involuntary contractions in the digestive tract, including the esophagus, stomach, and most of the intestines. The somatic sensory fibers of the VN receive sensory information from the skin behind the ear, the external part of the ear canal, and certain parts of the throat, whereas the visceral sensory ones convey the sensory input from the larynx, esophagus, lungs, trachea, heart, and most of the digestive tract. Taste near the root of the tongue travels through the special sensory fibers of the VN [47].
The GN and VN are thinner than the trigeminal nerve; the mean diameter of the formers are about 1.2±0.3 mm, whereas that of the latter is 2.84 mm, and it can get up to 9.7 mm (4.6– 9.3) as the trigeminal ganglion in the Meckel’s cave [1,48]. The length of the GN in the cistern is estimated as 16.2±1.9 mm, while that of the trigeminal nerve is 9.66 ±1.71 mm [14,49]. The literature reads that among 5–6 rootlets of the VN, the proximal 2–3 are sensory branches while the remaining 2–3 are motor ones [33]. However, a research on anatomy of VN based on electrophysiological investigation revealed that ‘purely sensory’ rootlets may exist only in 50% of the subjects, while the remaining 50% showed ‘motor responses’ via all rootlets of the VN [19]. Given that the GN and VN are thinner and longer than the trigeminal nerve, the former might be more prone to iatrogenic injury during MVD. Also, the REZ of the glossopharyngeal-vagus complex is positioned caudal to that of the trigeminal nerve, which demands a craniotomy for GPN to be extended further toward the skull base. These anatomical features of the GN might present additional challenges for neurosurgeons when performing MVD.
A compression on the root entry zone (REZ) of the GN by a vessel or vessels has been acknowledged as the cause of GPN in the great majority of cases [7,16,20]. Several cases of secondary GPN have been reported : tumor in the cerebellopontine angle, infections, multiple sclerosis, Paget’s disease, trauma, dental procedure, etc. [18,36,50]. A true idiopathic GPN where no cause was demonstrated around the GN appears to be extremely rare. Given the rarity of GPN, there are no precise data on how many percent of GPN are attributed to neurovascular compression. Our own observation demonstrated that 76 of 78 cases were caused by the compression by a vessel or two on the REZ of the GN alone or both GN and VN. There were two cases where no definite offending artery was found around the REZ during MVD, but one could not automatically conclude that they were completely idiopathic. In one of the two subjects, we speculate that the arachnoid membrane had been tethering the exceptionally tortuous posterior inferior cerebellar artery (PICA) to the REZ, yet upon the release of the arachnoid membrane, the PICA was no longer present at the REZ. The fact that the patient became symptom-free following the surgery where only wide dissection of the arachnoid membrane was performed could support our assumption. As for the other one, although there was no artery compressing the REZ, small arterioles did exist around it. During the surgery, instead of the conventional MVD, the arterioles were simply detached from the REZ of the GN, following which the patient became symptom-free, and no recurrence has been reported until now for more than 3 years. We suspect that some of idiopathic GPN could have been named so due to incomplete exploration around the GN. A thorough investigation around both the GN and VN during MVD is crucial to accomplish a cure, since a decompression only on the GN may result in remaining vagal symptoms such as swallowing difficulty or paroxysmal cough with or without GPN.
Vascular compression on the REZ in GPN, as in hemifacial spasm (HFS) or TN, may lead to microscopic disruption of the integrity of myelination, and then, in turn, ephaptic transmission in the nerve fibers [3,32]. Although it is still undetermined whether or not the vascular compression per se could alter the structure in the nucleus related to the GN, the demyelination and consequential ephaptic conduction resulting in neural hyperexcitability appears to be the proper etiology for GPN. Relatively high success rate (higher than 90%) of MVD for GPN also advocates this theory [7,31].
Combination of TN, HFS, and GPN or subparts of them is referred to as hyperactive dysfunction syndrome (HDS) of cranial nerves; according to large cohort studies among HFS patients, the prevalence of HDS was reported as up to 3% [25,53]. The only statistically proven risk factor for HDS was the advanced age that may be associated with atherosclerosis in multiple vessels [53].
DIAGNOSIS
Precise diagnosis of GPN is imperative; once it is properly done, physicians should be able to provide its natural course, treatment options, possible complications and prognosis, which this article seeks to address. Diagnosis of GPN, the key to successful treatment, must be based on clinical symptoms. The symptoms, characterized by unilateral brief paroxysmal lancinating pain in the sensory distributions of the GN and sometimes VN (Table 1) [55]. The distribution of the GN is defined as the posterior part of the tongue, tonsillar fossa, pharynx or angle of the lower jaw and/or in the ear [16]. More than half of the patients with GPN also complain a radiating neuralgic pain to the deep ipsilateral ear, although they often find it difficult to locate it without being inquired.
Vagal manifestations such as bradycardia, syncope, hypotension or cardiac arrest, are reported to be found approximately in 10% of GPN patients [2,3,16,17]. If the term ‘vagal manifestation’ refers to responses via visceral sensory component of the vagal nerve, we also have encountered those vagal manifestations in patients of our series : swallowing difficulty, hoarseness, or paroxysmal cough while talking or drinking, developed separately or together with the pain attack. Considering that the pain in the deep throat, one of the most common symptoms of GPN, can be accounted for by both the GN and somatic sensory component of the vagal nerve, we advocate the term VGPN [40].
The GPN pain can also radiates to other areas including the eye, nose, chin or shoulder, which mimics some symptoms of TN. Also, GPN and TN may share the same triggering actions including chewing, talking or brushing teeth, but the distribution of the former must include the throat, deep ear or deep neck, which the latter generally does not involve. Therefore, the symptoms of GPN must be TN or other various atypical facial pain syndromes, as well as dental diseases. Differentiation between GPN and TN is not always straightforward, because parts of the distribution of the glossopharyngeal nerve may overlap with that of the mandibular branch of the trigeminal nerve.
GPN can be misdiagnosed as TN, and vice versa. Therefore, an elaborate and insightful history taking is the key to the correct diagnosis; also, one must not overlook the possibility of coexistence of both diseases. Patients’ verbal complaints are often limited to the most severe symptom even when they have both TN and GPN, which can allow one of them neglected. It must be reminded that diagnosis of one of them does not automatically rule out the other, and symptoms related to the other must be inquired as well, since some patients may need two separate MVDs. When a patient suffers from both GPN and TN, the intensity of GPN can be stronger than that of TN, which may allow omission of diagnosis of TN. Reversely, if an individual with both GPN and TN experiences typical TN symptoms along with GPN symptoms in its initial stage, diagnosis of GPN can be overlooked. As for GPN with continuous background pain, i.e., atypical GPN, there is no guarantee that MVD is to provide a cure for them, although in limited cases where an atypical GPN is responsive to carbamazepine, MVD was reported to be effective in alleviation of pain [28].
The importance of accurate diagnosis cannot be over emphasized in achieving successful treatment for GPN. As the aforementioned GPN patient reported in 1910 had been misdiagnosed as TN, we also misdiagnosed our first GPN patient who visited our clinic. The patient complained typical pain suggesting GPN, but received MVD for TN based on a misdiagnosis. Needless to say, the MVD for TN failed to yield pain relief. When the patient was re-inquired afterwards about the symptoms with much greater detail, we realized that the pain that he had described was ignored by us during the initial encounter. On a later consideration, the patient also had a radiating pain to the ipsilateral deep ear which can be an important diagnostic clue for GPN. A second MVD was performed for GPN and it resulted in complete cure.
Lessons we learned from the diagnostic failure of the first patient are as follows : 1) possibility of GPN need always to be considered; 2) diagnosis of GPN must be made only whenever we meet the patient with severe facial pain including TN, and vice versa; 3) both typical TN and typical GPN may be triggered by coughing, talking, chewing or swallowing; the absence of these precipitation factors can indicate atypical GPN or atypical TN. What can separate GPN and TN is always the distribution of the pain, not the pain characteristics or precipitation factors; 4) the tongue base is where innervation by the GN overlaps with that by the trigeminal nerve. When patients’ main symptom is located in the tongue base, other accompanying symptoms must be inquired; concomitant pain in the deep throat may indicate GPN, whereas that in the face may be consistent with TN; and 5) coexistence of both TN and GPN should also be in consideration.
TREATMENT
GPN, at least during the early stage, tends to be responsive to medications, but for those whose GPN symptoms are medically intractable, advantages and disadvantages of various surgical options should be offered : glossopharyngeal rhizotomy with or without partial VN rhizotomy, percutaneous radiofrequency thermocoagulation, trigeminal tractotomy, MVD, and Gamma Knife radiosurgery (GKRS). Among them, MVD surgery is the only curative and non-destructive treatment modality for GPN, whereas all other surgical methods are more or less destructive, yet not aimed to be curative.
Pharmacological treatment
Upon diagnosis of GPN, medications that are proven to be effective for TN can also be applied to GPN, and the goal of the pharmacologic treatment is to alleviate the neuropathic pain, rather than to attempt a cure. Carbamazepine (100– 2000 mg), as it was reported to yield 100% pain relief in 70% of TN patients during the initial application, should also be the first one to be tried for GPN [51]. Other regimens recommended by the International Association for the Study of Pain (IASP) include gabapentin (100–5000 mg), pregabalin (75– 500 mg), valproic acid (125–2500 mg), lamotrigine (50–500 mg), et al. [9,27,39]. The optimal dose should be individually titrated and adjusted. With the proper application of medicine, GPN pain would most often show ‘relapsing and remitting’ pattern, then eventually reach ‘tolerable’ state, in about 2 months after the initiation of medical treatment [42]. This ‘tolerable’ state may last several months or years, but often the pain returns and becomes intolerable despite the use of increased dose, different agents or combination regimens. Adjuvant medications include selective serotonin reuptake inhibitors, vitamin B12, or opioids [42]. When combined with cardiovascular presentation, atropine can be administered, although it does not lessen the pain.
MVD
MVD is a marvelous product of modern microscope-assisted surgical techniques and anatomical researches demonstrating the neuro-vascular relationships; since it was successfully applied to TN and HFS by Laha and Jannetta [20] in 1970, it has become the treatment of choice for both diseases [11,13,31]. Encouraged by the satisfactory results of MVD for TN and HFS, Laha and Jannetta [20] employed it for GPN as well in 1977. According to a review of the literature by Chen and Sindou [7] in 2014, the total relief rate was reported between 50% and 100%, yet the authors suggested that the results seemed to be largely dependent on the level of equipment, surgeons’ experience, and the use of neurophysiological monitoring whether or not the procedure.
Decompression on the REZ of the GN and VN begins with a separation from the compression vessel, and ultimately from the pulsatile signal from it. Then, the physical separation needs to be maintained to achieve the long-term cure. MVD can be a safe and efficient procedure for GPN as much as for TN or HFS with the success rate higher than 90%, in a wellequipped facility. Surgical difficulty might be more associated with existence of perforators originating from the offending artery and the location of the perforators which make a longterm maintenance of decompressed state of the causative artery difficult. In the author’s institution, all MVDs were performed by a single surgeon (Y.A.) with use of computed tomography (CT) -guided navigation system for suboccipital craniotomy (Fig. 1). CT navigation-guided craniotomy was used to expose the margin of the sigmoid sinus from its beginning to the region behind the mastoid tip and to reduce the risks of bleeding from the sigmoid sinus. Intraoperative brainstem auditory evoked potentials and sensory evoked potentials (Viasys Healthcare, Conshohocken, PA, USA) were monitored throughout the operation to check the function of the brainstem and cranial nerves.
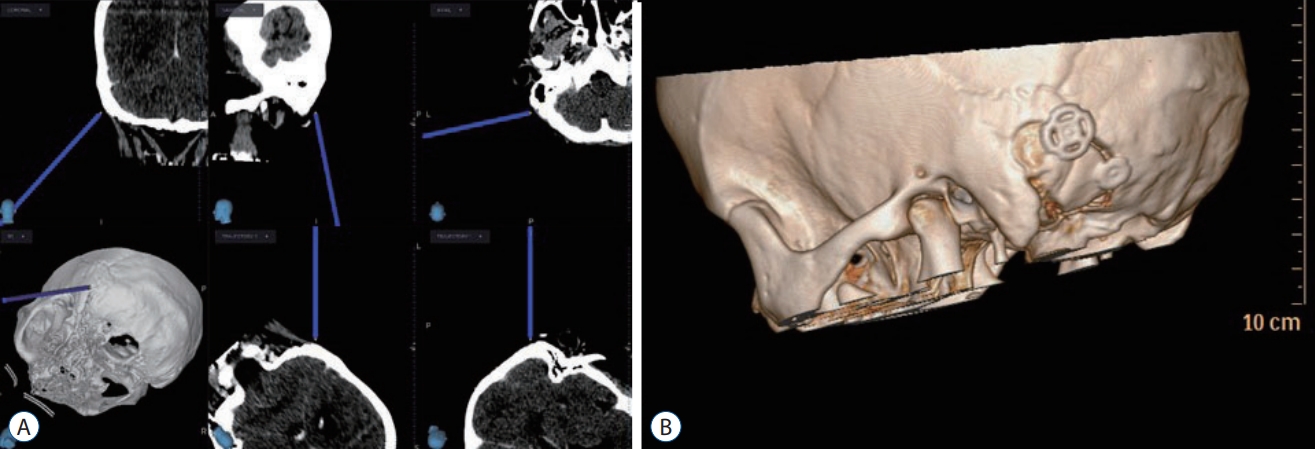
A : Computed tomography (CT) guided navigation for identification of the sigmoid sinus and mastoid air cells. B : Postoperative 3D CT image showing the size and shape of the suboccipital craniotomy.
The lateral suboccipital infra-f loccular approach with a park-bench position was employed as this approach is enough to achieve a wide surgical field [37]. A more extensive craniotomy, e.g., transcondylar fossa approach has not been proven to be more beneficial than conventional lateral suboccipital one in overcoming the limitation associated with perforating arteries. The extradural tissues were covered with saline-soaked gauze to avoid tissue dry-up during the surgery. A C-shaped dural incision was made and reflected over the sigmoid sinus. The arachnoid membrane was dissected carefully to allow cerebrospinal fluid (CSF) drainage and to expose the lower cranial nerves and the vessels responsible for GPN. A retractor with a 2-mm-wide tip was applied intermittently during the arachnoid dissection, if needed. A wide and careful arachnoid dissection was carried out to avoid any retraction or injury from arachnoid. Several pieces of Tef lon (Bard PTFE felt, Tempe, AZ, USA) and a Tisseel glue (Baxter Healthcare Corp., Glendale, CA, USA) were prepared as a pre-filled syringe. Upon visualization of REZ of the GN, the decompression was carried out using one of four surgical techniques depending on the surgical finding : simple transposition of the vessel using Teflon pieces (Fig. 2), transposition of the vessel using one or two glue-coated Teflon slings (Fig. 3A and B); interposition of Tef lon pieces underneath the offending vessel or vessels (Fig. 4), and cauterization of offending veins [16]. We have adopted ‘transposition’ technique since 2014 [16]. Instead of traditional interposition technique where Teflon felt is positioned ‘on’ the REZ, this transposition technique involves moving the offending vessel ‘off’ the REZ, and when Teflon pieces are used, they are also positioned ‘off’ the REZ. According to our previous report with 30 cases of GPN, the most commonly performed technique was simple transposition with Tef lon pieces, followed by transposition using a glue-coated Teflon sling and interposition of Tef lon pieces in that order (Table 2) [22]. Cauterization of the vein was applied for one case. No additional rhizotomy was accompanied in our series.
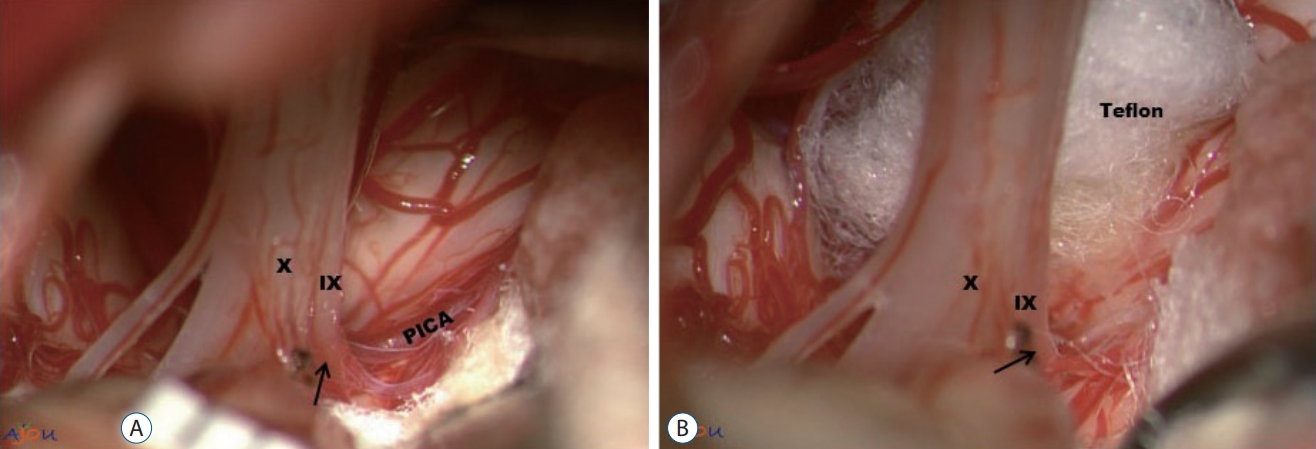
Simple transposition using Teflon pieces. A : Posterior inferior cerebellar artery (PICA) is compressing the root entry zone (REZ) (arrow) of the IX and X nerve. B : The position and trajectory of PICA is altered by the inserted Teflon. PICA is now moved behind Teflon (not visible in the picture. Also, note that Teflon is positioned 'off' the REZ). Since Teflon is not used as an insulation material on the REZ, we define this technique 'transposition'. IX : the glossopharyngeal nerve, X : the vagus nerve.
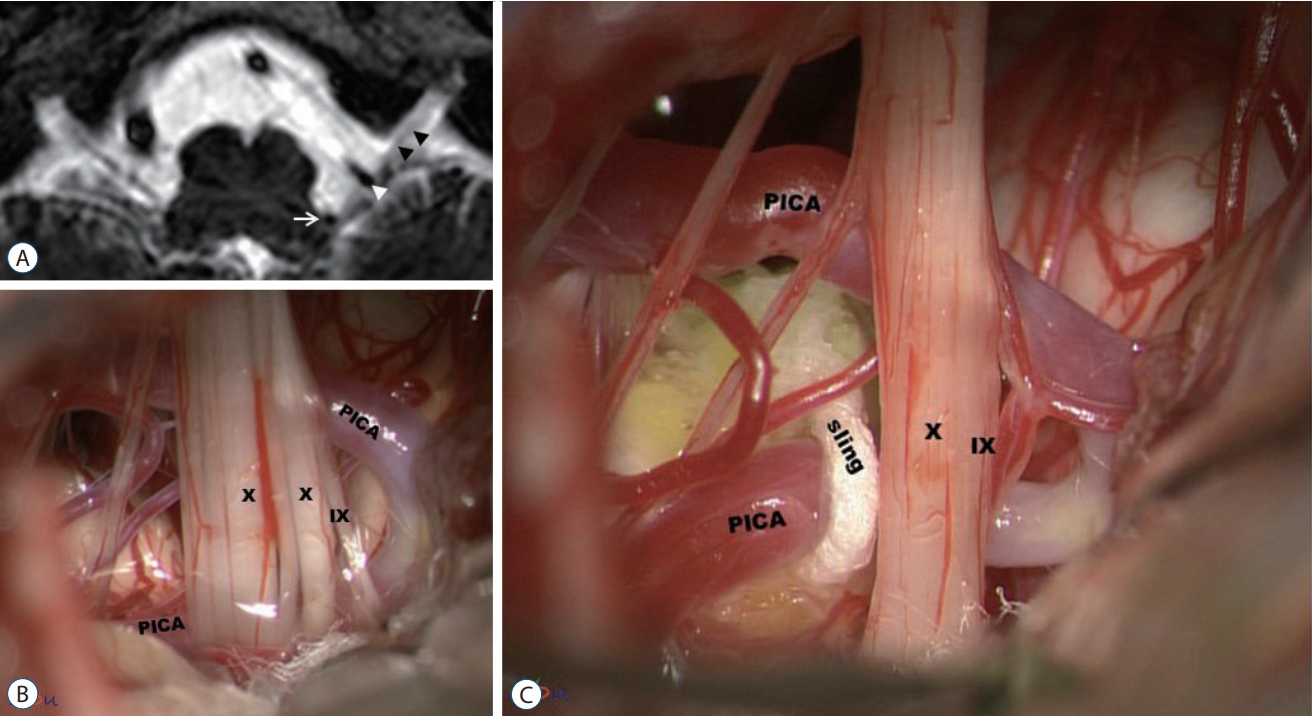
Transposition using a Teflon sling. A : Very tortuous posterior inferior cerebellar artery (PICA) is compressing the root entry zone (REZ) of the IX and X nerves (white arrow : proximal PICA compressing the REZ of the CN IX-X complex; white arrowhead : distal PICA compressing the cisternal portion of the CN IX-X complex; black arrowheads : the CN IX-X complex). B : Tortuous PICA compressing the REZ and the cisternal portion of the CN IX-X complex. C : A fibrin-glue coated sling is pulling PICA away from the REZ, resulting in a change of the position and tracjectory of PICA. Through this 'tranposition' technique, both the REZ and cisternal portion of the CN IX-X complex can be decompressed. IX : the glossopharyngeal nerve, X : the vagus nerve.
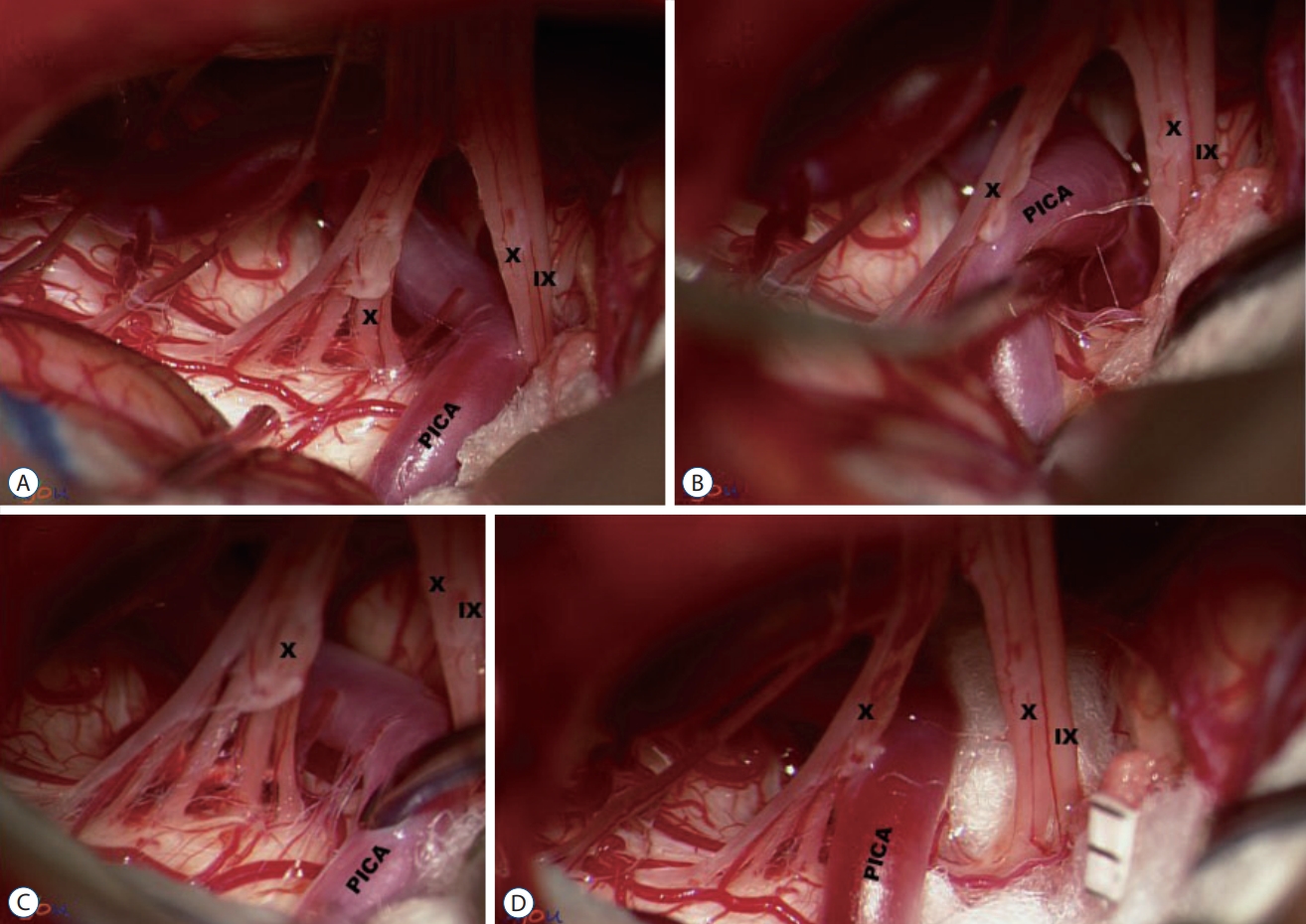
Interposition of insulating material (IM). A : Posterior inferior cerebellar artery (PICA) compressing the IX nerve. B and C : while PICA being maneuvered, it appears unfeasible to transpose PICA to another position, owing to several perforators tethering PICA to the brain stem. D : Teflon pieces are inserted as an IM. IX : the glossopharyngeal nerve, X : the vagus nerve.
Transposition using Teflon pieces
When Laha and Jannetta [20] originally introduced this technique of inserting insulating materials (IMs) between the offending artery and the REZ for TN, HFS, and GPN, they addressed this as ‘interposition’. In our opinion, it can better be named ‘transposition’ than ‘interposition’, when the insertion of IM causes the vessel securely displaced from its original position, i.e., on the REZ. A mere interposition of IM and its insulating effect per se, with no definite change of force vector from the offending vessel, in our opinion, cannot explain the whole mechanism of decompression. In ‘transposition’ technique, Teflon pieces are inserted not to become an insulation material, but to change the position and direction of the compressing vessel. Therefore, the REZ can be released not only from the physical compression by the offending vessel, but also from the pulsatile force from it, because the force vector can be re-directed off the REZ. Transposition technique can be characterized as ‘off the REZ’, since the offending vessel is moved away from the REZ, and when Teflon pieces are used to maintain the ‘off the REZ’ state, they were also placed ‘off the REZ’. This ‘off-the-REZ’ policy depicts the essence of the ‘transposition’, and we believe this could provide a foundation for prevention of recurrence, in that the REZ of both the GN and VN is free from any compression, not only by the offending vessel, but also by Tef lon pieces. Although Tef lon is known to be chemically stable, it has not been proven whether it remains stable while being in contact with pulsating CSF for several decades.
We propose that the term interposition can better be used for occasions where the interposed Teflon pieces merely insulate the REZ, changing neither position nor force vector of the vessel. In our series, transposition using Teflon pieces and fibrin glue was always attempted with a few exceptions.
Transposition using a fibrin glue coated Teflon sling
If the compressing vessels are tortuous, atheromatous, or large in diameter, a mere transposition using Tef lon pieces may not be sufficient to achieve a long-term decompression. To bring and keep the vessel away from the REZ, a glue-coated Teflon sling was used. This sling was applied to change the direction of the vessel so that the REZ can be freed from any compression, and then, it was secured to a non-movable structure, e.g., the petrosal dura or the top of the brainstem in our series [22]. For this procedure, a glue-coated Tef lon sling were hand-crafted by the surgeon, using small pieces of glue-coated Teflon (Fig. 5). Several Teflon threads were manually twisted and combined with help of fibrin glue until it formed an elongated strand. The length and thickness of the strand, i.e., the sling, was modified in accordance with each individual’s anatomical feature. One should be reminded that the sling never has to be thicker than necessary, for an excessively thick sling may complicate the procedure by taking up a limited work space or by being an iatrogenic source of compression on the REZ, because the cerebellum, after being released from the retraction, may move the sling, and an excessively thick sling may contribute to an additional compression on the REZ. The sling was positioned to encircle the offending artery, and then the ends of it were fixated to a place ‘off the REZ’ which included the petrosal dura or on the top of the brain stem using fibrin glue or fibrin glue plus TachoSil® pieces. When fibrin glue and TachoSil pieces were expected to be insufficient to secure the sling to the constant part of the brain, additional fixating procedures including one or two stitches to the petrosal dura were carried out (Figs. 2-5). In addition, Teflon pieces could also be used to support the Teflon sling, and they, too, should be placed ‘off the REZ’.
Interposition of IM
The true interposition was performed only when the offending vessel failed to be manipulated enough to be transposed off the REZ [16,22]. In five of 78 cases in our series, short perforating arteries from the offending artery were tightly tethering it to the REZ, which precluded or limited the application of transposition; hence, the only available option left is the interposition. Unlike the ‘transposition’ technique, interposition of IM solely contributed to decompression of the REZ with minimal alteration of the position or course of the vessel. This technique was reserved only for arteries with a smaller diameter, and accordingly, less pulsatile force on the REZ of GN and VN.
Cauterization of vein
On rare occasions, a thorough investigation around the REZ of GN and VN failed to locate a compressing artery, although several engorged veins appeared to compress the REZ. Considering that venous compression as the sole cause of TN was reported to be 6-18%, the same etiology can contribute to GPN [26,29]. A vein or veins were thought to be the cause of neurovascular conflict in three of 78 GPN patients in our series. As the veins were profusely anastomosed with other collateral ones, the engorged portion of the vein on the REZ was cauterized and divided, using the lowest possible power setting of the bipolar coagulator. All patient became symptom-free without a neurologic deficit after the cauterization of the vein, and no hemorrhagic or ischemic event was accompanied.
Since no cohort-based statistics are available on the success rate of transposition procedure-based MVD for GPN, we present our own results. A total of 78 typical GPN patients underwent MVD in our institute for the past two decades, and all had a follow-up of at least 6 months. The Barrow Neurological Institute (BNI) pain intensity score was used for evaluation of the pain. Seventy-six (97.4%) of 78 patients became pain free (BNI grade 1) or with only occasional pain (BNI grade 2) after MVD. There were 49 GPN patients with a longer follow up (more than 5 years); 48 (97.9%) of them became BNI 1 or 2 in 1 year after MVD. On a chronological analysis, eight of those 49 patients had stated that their pain belonged to BNI grade 3 after MVD, but seven of them became BNI grade 1 or 2 in 1 year. One individual has remained in BNI grade 3 and she, despite her general satisfaction with the result, still periodically needed medicines for the pain especially in the winter and when seasons were changing. While there were three patients who experienced transient complications, such as dysphagia, dysphonia, and decreased hearing ability, neither mortality nor permanent complication occurred in our series. Also, no VN-related complication took place.
Considering that the success rate of MVD for TN (80% to 85%) reported by cohort studies is somewhat lower than that for HFS (90%), we cautiously speculate that MVD might be more beneficial to GPN than to TN [6,23,30]. The relatively high success rate of MVD for GPN might be attributed to the anatomical simplicity of the 9th and 10th cranial nerves, and no bony structure to encompass it except for the jugular foramen. In such sense, it is probably more likely for a successful MVD to provide a long-term cure for GPN than for TN. However, revision surgeries for GPN should be avoided at all costs, given its anatomical features. Compared to the trigeminal nerve, the GN and VN are much thinner and a hypothetical injury to them may cause irreversible, sometimes life-threatening consequences. Therefore, we suggest that the ‘transposition’ technique should always be aimed for, as the mere ‘interposition’ cannot guarantee a long-term cure. Regardless of the kind of surgical techniques, perforating arteries must not be jeopardized, and maneuvering the vessels should be limited to the minimum in order to prevent vasospasm.
Selective rhizotomy
When Dandy [8] first performed intracranial glossopharyngeal nerve rhizotomy (GNR) in 1927, he reported that the immediate result was good, but there were frequent recurrences. To improve the long term result, GNR started to include an additional rhizotomy of the sensory branches of the VN [4]. Complications regarding partial rhizotomy of the upper rootlets of the VN are thought to be benign (irritative cough, foreign body sensation in the throat, hoarseness, dysphagia, etc.). Yet a review article with 454 MVD and 157 GNR cases reported that permanent complications after GNR (19.1%) was three times greater than those after MVD (5.1%) [34].
Another research regarding selective GNR with or without partial VN rhizotomy in 103 patients with a follow up of 2.3 years reported that the pain relief and immediate complications did not significantly differ between the two groups, while the long term complications after GNR with partial VN rhizotomy (35.8%) were much more frequent than those after GNR without VN rhizotomy (3.8%) [24]. The authors concluded that GNR, especially without VN rhizotomy, can be a safe and effective treatment option for GPN, yet we respectfully could not concur with their conclusion, given a relatively short follow up (2.3 years) and the high rate of long-term complications after GNR with partial VN rhizotomy. Furthermore, a research on anatomy of the VN using electrophysiological investigation suggested that the prevailing knowledge that the proximal 2–3 branches were sensory and the remaining 3–4 ones were motor rootlets may not be entirely true; they revealed that in 50% the subjects, there were no ‘purely sensory’ rootlets, and in the remaining 50% who possess ‘purely sensory’ rootlets, they were most likely confined in only one most rostral branch, not in 2–3 proximal ones [19]. Supporting their findings, we strongly object to a ‘blind’ VN rhizotomy, no matter how partial they can be, unless a very thorough and careful electrophysiological study precedes the destruction of a part of the VN.
Starting from Lazorthes’ first report, percutaneous radiofrequency thermocoagulation was attempted by several authors, yet it did not become a popular procedure mainly because it, by its nature being a blind procedure around the VN, jugular vein and internal carotid artery, has potential to cause various complications including dysphagia, vocal cord paralysis, or even cardiovascular events [10,21,41,46]. In our opinion, there is no need to add an adjuvant rhizotomy during MVD for GPN. We believe that MVD with the ‘off the REZ’ policy alone is the best treatment for GPN, in that it is non-destructive and can afford the cure.
GKRS
Inspired by the application of GKRS for TN for the past two decades, Stieber [45] reported the first case of GKRS for GPN in 2005. Following subsequent reports on application of GKRS for GPN, two multicenter-based studies are currently available, and we believe they are worth mentioning [5,15,44,52,54]. An international multicenter study, where 22 GPN patients had undergone GKRS from six academic medical centers, was published in 2016; a single 4-mm isocenter was targeted to the GN nerve at the level of the GN meatus, then the radiation was delivered with the median maximum dose of 80 Gy (range, 80–90 Gy) [15]. The authors reported 50% (11 of 22) of complete pain-relief (BNI grade 1) and 73% (16 of 22) of favorable outcome (BNI grade 1, 2, and 3) in 3 months following the radiation, although the maintenance of the favorable outcome was estimated 28% in 7 years. Eight (50%) of 16 patients who initially responded favorably to GKRS experienced a recurrence. Persistent hypesthesia in the palatoglossal arch was reported as a complication in two patients, and both took place after a second GKRS. Another bi-center study involving 21 subjects reported in 2018 that 17 (81%) became pain-free after the initial radiosurgery, but 10 (58.8%) of them experienced a recurrence in around 13 months [5]. Overall, 17 patients (80.9%) were pain-free at the last follow up (mean follow-up, 5.2 years), and there was one complication noted : a transient paresthesia of the edge of the tongue. Given that the recurrence following initial improvement was not exceptional in both reports, candidates for GKRS should be informed that they may need another session of GKRS or a different treatment modality.
GKRS can be acceptable as an alternative treatment for GPN when a patient is ineligible for MVD. Since December 2021, The Korean National Health Insurance has approved a radiosurgery for treatment of limited cases of GPN. Although GKRS may not guarantee an instant cure for GPN, one cannot deny that it can substantially decrease the intensity and frequency of the neuralgic pain. Provided that patients are fully aware of the fact that they may need another session or more, as well as continuation of medication, GKRS can be a better option than rhizotomy, based on the reasons as follows : 1) GKRS is minimally invasive and requires no general anesthesia, 2) no mortality has been reported thus far, 3) their reported morbidity was minor and transient, and 4) repeated sessions do not necessarily increase the procedural risk.
CONCLUSION
GPN, a cranial nerve rhizopathy, belongs to neurovascular compression syndromes. Symptoms of GPN must be carefully evaluated and differentiated from other pain syndromes, especially TN. MVD should be considered as the primary choice of treatment aimed to cure the GPN, and ‘off-the-REZ’ technique, if applicable, should be attempted during MVD, for it can fundamentally prevent recurrence. GKRS can be an alternative option when a patient is ineligible for MVD, though it is categorized as a destructive procedure.
Notes
Conflicts of interest
No other potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : YHA; Data curation : YHA; Formal analysis : YHA; Methodology : JSP, YHA; Project administration : YHA; Visualization : YHA; Writing - original draft : JSP; Writing - review & editing : JSP, YHA
Data sharing
None
Preprint
None