The Clinical Characteristics of Electrolyte Disturbance in Patients with Moderate and Severe Traumatic Brain Injury Who Underwent Craniotomy and Its Influence on Prognosis
Article information
Abstract
Objective
The present study aimed to investigate the clinical characteristics of electrolyte imbalance in patients with moderate to severe traumatic brain injury (TBI) who underwent craniotomy and its influence on prognosis.
Methods
A total of 156 patients with moderate to severe TBI were prospectively collected from June 2019 to June 2021. All patients underwent craniotomy and intracranial pressure (ICP) monitoring. We aimed to explore the clinical characteristics of electrolyte disturbance and to analyze the influence of electrolyte disturbance on prognosis.
Results
A total of 156 patients with moderate and severe TBI were included. There were 57 cases of hypernatremia, accounting for 36.538%, with the average level of 155.788±7.686 mmol/L, which occurred 2.2±0.3 days after injury. There were 25 cases of hyponatremia, accounting for 16.026%, with the average level of 131.204±3.708 mmol/L, which occurred 10.2±3.3 days after injury. There were three cases of hyperkalemia, accounting for 1.923%, with the average level of 7.140±1.297 mmol/L, which occurred 5.3±0.2 days after injury. There were 75 cases of hypokalemia, accounting for 48.077%, with the average level of 3.071±0.302 mmol/L, which occurred 1.8±0.6 days after injury. There were 105 cases of hypocalcemia, accounting for 67.308%, with the average level of 1.846±0.104 mmol/L, which occurred 1.6±0.2 days after injury. There were 17 cases of hypermagnesemia, accounting for 10.897%, with the average level of 1.213±0.426 mmol/L, which occurred 1.8±0.5 days after injury. There were 99 cases of hypomagnesemia, accounting for 63.462%, with the average level of 0.652±0.061 mmol/L, which occurred 1.3±0.4 days after injury. Univariate regression analysis revealed that age, Glasgow coma scale (GCS) score at admission, pupil changes, ICP, hypernatremia, hypocalcemia, hypernatremia combined with hypocalcemia, epilepsy, cerebral infarction, severe hypoproteinemia were statistically abnormal (p<0.05), while gender, hyponatremia, potassium, magnesium, intracranial infection, pneumonia, allogeneic blood transfusion, hypertension, diabetes, abnormal liver function, and abnormal renal function were not statistically significant (p>0.05). After adjusting gender, age, GCS, pupil changes, ICP, epilepsy, cerebral infarction, severe hypoproteinemia, multivariate logistic regression analysis revealed that hypernatremia or hypocalcemia was not statistically significant, while hypernatremia combined with hypocalcemia was statistically significant (p<0.05).
Conclusion
The incidence of hypocalcemia was the highest, followed by hypomagnesemia, hypokalemia, hypernatremia, hyponatremia and hypermagnesemia. Hypocalcemia, hypomagnesemia, and hypokalemia generally occurred in the early post-TBI period, hypernatremia occurred in the peak period of ICP, and hyponatremia mostly occurred in the late period after decreased ICP. Hypernatremia combined with hypocalcemia was associated with prognosis.
INTRODUCTION
Traumatic brain injury (TBI) has been the leading cause of death or disability in young people [10]. Although the therapeutic level of TBI has been greatly improved due to the development of medical technology, the treatment of severe TBI is still difficult, with multiple types of complications, causing high disability rate and death rate [8]. Electrolyte disturbance, including hypernatremia, hypokalemia and hypocalcemia, are common in moderate and severe TBI, which will affect the prognosis of patients if not timely corrected. Most of the existing literature only focuses on the disturbance of single ions, such as sodium, potassium and calcium. Although a small number of studies focus on the overall electrolyte disturbance of patients with TBI [34], the rules of occurrence and development of various electrolyte disturbance and the influence on prognosis are still unclear. In this article, we investigated various electrolyte disturbance and the influence on clinical outcome of patient with TBI.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of our hospital, and all patients or their family members signed written informed consent.
Study subject
TBI patients were prospectively selected from June 2019 to June 2021, who were admitted to The Second Affiliated Hospital of Jiaxing University (IRB No. JXEY-2022SW011). The inclusion criteria were as follows : 1) patients with moderate to severe TBI (Glasgow coma scale [GCS] ≤13 points); 2) aged 18 to 75 years; 3) no severe underlying disease; 4) undergoing craniotomy and intracranial pressure (ICP) monitoring; and 5) survival ≥1 week. The exclusion criteria were as follows : 1) patients with a history of adrenal glands, thyroid, etc. that may cause electrolyte disturbance and 2) patients with severe damage to other organs (injuries to other organs affect the lives of patients or require emergency surgery), or TBI patients who have received cardiopulmonary resuscitation.
Therapeutic approaches
All patients were treated with neurosurgery intensive treatment plan according to the individual study protocols, but largely based on the guidelines for treatment of severe TBI at the time. All patients underwent craniotomy and ICP monitoring within 24 hours after injury. None of the patients were treated with hypertonic saline to decrease ICP.
Observational indicators
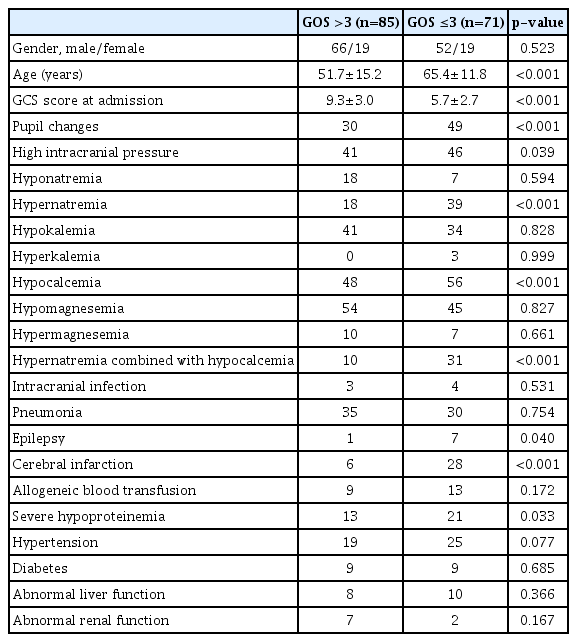
The following parameters of patients were recorded, including age, gender, GCS score at admission, pupil changes, ICP, intracranial infection, pneumonia, epilepsy, cerebral infarction, allogeneic blood transfusion, severe hypoproteinemia, hypertension, diabetes, abnormal liver function, and abnormal renal function. Electrolytes were monitored at least once a day during the first week and then at least once every 2 days from 1 week to 2 weeks. Patients were followed up for 0.5 years to investigate the clinical characteristics of electrolyte disturbance and its influence on prognosis in TBI patients. Several definitions were as follows : hyponatremia, serum sodium level <135 mmol/L; hypernatremia, serum sodium level >145 mmol/L; hypokalemia, serum potassium level <3.5 mmol/L; hyperkalemia, serum potassium level >5.5 mmol/L; hypocalcemia, serum calcium level <2.0 mmol/L ; hypomagnesemia, serum magnesium level <0.75 mmol/L; hypermagnesemia, serum magnesium level >1.02 mmol/L; severe hypoproteinemia, serum protein <25 g/L; high ICP, ICP ≥20 mmHg; good prognosis, Glasgow outcome scale (GOS) score >3; poor prognosis, GOS score ≤3.
Statistical analysis
SPSS ver. 17.0 software (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Measurement data with normal distribution were shown as mean±standard deviation, and t-test was used for comparison. Counting data were shown as the number of cases (percentage), and chi-squared test was used for comparison. Logistic regression analysis was used to adjust for other possible influencing factors to assess the correlation between electrolyte disturbance and prognosis. p<0.05 was considered as statistically significant.
RESULTS
General information
A total of 156 eligible TBI patients were enrolled, including 118 males and 38 females, with the age ranging from 18 to 75 years (average age, 57.9±15.3 years). There were 21 cases of open injuries and 135 cases of closed injuries. For GCS score at admission, there were 63 cases with 9–13 points, 36 cases with 6–8 points, and 57 cases with ≤5 points. There were 79 cases with pupil changes, including 56 cases with dilated pupils on one side, and 23 cases with dilated pupils on both sides. All patients underwent craniotomy, including 111 cases undergoing unilateral craniectomy decompression, 25 cases undergoing bilateral craniectomy decompression and 20 cases undergoing skull reintroduction.
The clinical characteristics of electrolyte disturbance
Of the 156 patients with severe TBI, there were 57 cases of hypernatremia, accounting for 36.538%, with the average level of 155.79±7.69 mmol/L, which occurred 2.2±0.3 days after injury. There were 25 cases of hyponatremia, accounting for 16.03%, with the average level of 131.20±3.71 mmol/L, which occurred 10.2±3.3 days after injury. There were three cases of hyperkalemia, accounting for 1.92%, with the average level of 7.14±1.30 mmol/L, which occurred 5.3±0.2 days after injury. There were 75 cases of hypokalemia, accounting for 48.08%, with the average level of 3.07±0.30 mmol/L, which occurred 1.8±0.6 days after injury. There were 105 cases of hypocalcemia, accounting for 67.31%, with the average level of 1.85±0.10 mmol/L, which occurred 1.6±0.2 days after injury. There were 17 cases of hypermagnesemia, accounting for 10.90%, with the average level of 1.21±0.43 mmol/L, which occurred 1.8±0.5 days after injury. There were 99 cases of hypomagnesemia, accounting for 63.46%, with the average level of 0.65±0.06 mmol/L, which occurred 1.3±0.4 days after injury. The detailed results were shown in Table 1.
Univariate regression analysis of the influence of electrolyte disturbance on prognosis
Univariate regression analysis revealed that age, GCS score at admission, pupil changes, ICP, hypernatremia, hypocalcemia, hypernatremia combined with hypocalcemia, epilepsy, cerebral infarction, severe hypoproteinemia were statistically abnormal (p<0.05), while gender, hyponatremia, potassium, magnesium, intracranial infection, pneumonia, allogeneic blood transfusion, hypertension, diabetes, abnormal liver function, and abnormal renal function were not statistically significant (p>0.05). The detailed results were shown in Table 2.
Multivariate analysis of the influence of electrolyte imbalance on prognosis
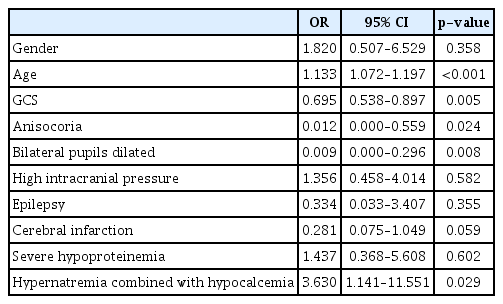
After Adjusting gender, age, GCS score, pupil changes, ICP, epilepsy, cerebral infarction and severe hypoproteinemia, multivariate Logistic regression analysis showed that hypernatremia combined with hypocalcemia was statistically abnormal (p<0.05), while simple hypernatremia or hypocalcemia was not statistically significant. The detailed results were shown in Table 3.
DISCUSSION
The incidence of electrolyte disturbance in TBI patients is high. It has been reported that the most common electrolyte disturbance in TBI patients is serum sodium among all types of serum electrolytes [1,30,34,36]. Among them, hyponatremia accounts for 4% to 51% [34,35,48]. Hyponatremia occurs from 2 days to 2 months after injury [4,39], mostly from 1 week to 2 weeks after injury [48]. The incidence of hyponatremia was low in our study, and the occurrence time of hyponatremia was 10.2±3.3 days. It is currently believed that central hyponatremia is common in cerebral salt wasting syndrome (CSWS) and syndrome of inappropriate antidiuretic hormone secretion (SIADH) [6], but the specific causes of CSWS and SIADH are still unclear [19], no cause has been found to be the only cause at present [19]. Hyponatremia can induce cerebral edema, leading to seizures and aggravating the condition. If it is not treated timely or properly, it can even endanger the life of the patients [19], which is an independent predictor of poor prognosis in TBI patients [37]. However, we failed to reveal any correlation between hyponatremia and poor prognosis in this group of cases.
Rafiq et al. [34] reported that the most common electrolyte disturbance in TBI was hypernatremia, accounting for 65.1%, followed by hyponatremia and hypokalemia. Pin-On et al. [31] also revealed that hypernatremia was more common than hyponatremia, and 86.3% of patients with severe TBI had hypernatremia. In our group, hypernatremia accounted for 37.2%, which was higher than hyponatremia. The average time of occurrence of hypernatremia was 2.2±0.3 days after injury, which was significantly earlier than that of hyponatremia. Pin-On et al. [31] found that patients with hypernatremia were older, which was consistent with our study. In our study, the average age of hypernatremia was 62.7±11.7 years, which was significantly older than the overall average age (57.9±15.3 years). It may be due to the decreased total water content in the body of elderly patients and women, who are, therefore, more susceptible to hypernatremia [17]. Hoffman et al. [14] and Wilcox [46] considered that hypernatremia was mainly caused by negative water balance, generally due to the water loss of kidney or gastrointestinal tract and sweating, occasionally accompanied by insufficient fluid intake or improper electrolyte solution treatment. Our cases have shown that hypernatremia is associated with intracranial hypertension, especially in patients with severe hypernatremia, which is often accompanied by severe intracranial hypertension. The average ICP in hypernatremia patients was 31.246±22.836 mmHg in our study. It remains controversially whether hypernatremia worsens the prognosis alone or is only a surrogate indicator of disease severity. On the one hand, the increased concentration of serum sodium can not only attenuate brain edema, but also regulate neuroinflammatory pathways, restore neuronal membrane potential as well as decrease blood viscosity [27,28]. On the other hand, hypernatremia can disrupt the balance of the body and may be detrimental to the body [21]. Hypernatremia can cause decreased glomerular filtration rate, leading to increased levels of creatinine and blood urea nitrogen [12,13,46]. Hypernatremia had also been confirmed to be associated with rhabdomyolysis [18], which had a negative effect on cardiac contraction [16] and can cause myelin lysis and cell necrosis. Additionally, rapid correction of hypernatremia may lead to ICP rebound, cerebral edema and seizures [9]. Many scholars have reported that hypernatremia after severe TBI is independently associated with poor prognosis and death [14,20,23,42,43]. Univariate analysis of this group revealed that hypernatremia was a risk factor for poor prognosis, while multivariate analysis showed that hypernatremia was not an independent risk factor for poor prognosis.
It has been reported that patients with TBI are more likely to develop hypokalemia than patients with other types of trauma [3,33]. Wu et al. [47] has revealed that the incidence of hypokalemia was 32.4%, and the peak of severe hypokalemia occurred in the first 24–96 hours. Pin-On et al. [31] has found that hypokalemia is the most common electrolyte disturbance in TBI patients, accounting for 65.5%. The incidence of hypokalemia in this group was 48.077%, which occurred 1.8±0.6 days after injury. Hypokalemia after TBI is caused by the massive release of catecholamines after TBI, which leads to the transfer of potassium ions from extracellular space to intracellular space [7,11,33]. The application of diuretics such as furosemide can cause hypokalemia, and mannitol can also increase urinary potassium excretion and cause hypokalemia [5]. Wu et al. [47] demonstrates poor prognosis in severe hypokalemia group, which is considered as an independent risk factor for death in TBI patients. However, our study failed to show any correlation between hypokalemia and poor prognosis.
Hypocalcemia after TBI is very common, with an incidence of 33–62.3% [34,44]. Our study showed that the incidence of hypocalcemia was 67.308%, which is the most common type of electrolyte disturbance in moderate to severe TBI. After TBI, due to the sudden influx of calcium ion into the cells, the level of extracellular calcium ions is decreased, resulting in hypocalcemia [24]. Traumatic deformation of the cell membrane [22], hyperphenylalanineemia [32], decreased serum magnesium concentration and hyperventilation to control the increased ICP [8] might all promote the entrance of calcium ions into cells, resulting in hypocalcemia. Extensive use of furosemide and mannitol increases calcium excretion, and colloid-induced hemodilution is also an important cause of hypocalcemia in severe trauma patients [45]. The increased content of intracellular calcium inhibits mitochondrial enzymes and activates lipase, playing a role in apoptosis [2]. Vinas-Rios et al. [44] considers that the rapid increase of intracellular calcium ions after TBI can trigger the cellular mechanism causing neuronal dysfunction and death. Manuel et al. [24] considers that hypocalcemia is a risk factor for poor prognosis. In our study, univariate analysis suggested that hypocalcemia was associated with poor prognosis, but multivariate analysis failed to reveal that hypocalcemia was associated with poor prognosis.
The incidence of hypomagnesemia in TBI patients is 5.6–58% [29,34,41]. Our data showed that hypomagnesemia was 63.462%, second only to hypocalcemia. The incidence of hypermagnesemia is relatively low, which is reported to be 2.8% by Rafiq et al. [34]. Our study demonstrated that hypermagnesemia was 10.897%. The mechanism of magnesium depletion in TBI patients remains unclear. The possible explanation is that stress induces sharply increased catecholamines to trigger an increase in lipolysis, leading increased free fatty acids bound to Mg2+, thereby increasing the urine excretion of Mg2+ [25,38]. Some scholars have also observed that the content of magnesium in the cerebrospinal fluid (CSF) was increased after TBI, but decreased in the serum [15,26,41]. Stippler M considers that blood brain barrier destruction can cause the penetration of serum Mg2+ into CSF, and Mg2+ of CSF may also originate from damaged brain cells secondary to hypoxia [41]. Stippler et al. [41] and Nayak et al. [29] believe that hypomagnesemia is associated with poor prognosis after TBI. In particular, when high magnesium of CSF and low magnesium of serum coexist after TBI, the prognosis will be significantly worse [15,41]. Our data show that abnormal magnesium ion is not associated with prognosis.
Univariate analysis of this group showed that the prognosis of patients with hypernatremia and hypocalcemia was poor. However, after adjusting for gender, age, GCS score, pupil changes, ICP, epilepsy, cerebral infarction, severe hypoproteinemia, hypernatronemia and hypocalcemia were not independent risk factors for poor prognosis of severe TBI, while hypernatremia combined with hypocalcemia, age, GCS score at admission and pupil changes were independent risk factors of prognosis in patients with severe TBI.
CONCLUSION
Electrolyte disturbances are common in patients with moderate and severe TBI. The incidence of hypocalcemia is the highest, followed by hypomagnesemia, hypokalemia, hypernatremia, hyponatremia and hypermagnesemia. Hypocalcemia, hypomagnesemia and hypokalemia generally occur in the early post-injury period, hypernatremia occurs in the peak period of ICP, and hyponatremia mostly occurs in the late period after decreased ICP. Hypernatremia combined with hypocalcemia is associated with poor prognosis. We suggest that dehydrating agents should not be used excessively, blood volume should be kept stable, and colloids should be used as little as possible. It is reasonable to regularly measure calcium concentrations and treat hypocalcemia [40].
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Author contributions
Conceptualization : GHW, HPS; Data curation : YY, ZC; Formal analysis : YY; Funding acquisition : GHW; Methodology : YY; Project administration : HPS; Visualization : HPS; Writing - original draft : GHW, HPS; Writing - review & editing : GHW, HPS
Data sharing
None
Preprint
None
Acknowledgements
This study was supported by Zhejiang provincial medical science and technology program (2022KY1257). The authors would like to thank Jiaxing Key Scientific and Technological Innovation Team--Targeted Drug Research and Tumor Nanotargeting and TCM Technology Innovation Team.