Spinous Process-Splitting Hemilaminoplasty for Intradural and Extradural Lesions
Article information
Abstract
Objective
To describe a novel spinous process-splitting hemilaminoplasty technique for the surgical treatment of intradural and posterior epidural lesions that promotes physiological restoration.
Methods
The spinous process was split, the area of the facet lamina junction was drilled, and en bloc hemilaminectomy was then performed. After removing intradural and posterior epidural lesions, we fitted the previously en bloc-removed bone to the pre-surgery same shape, and held it in place with non-absorbable sutures. Surgery was performed on 16 laminas from a total of nine patients between 2011 and 2014. Bony union of the reconstructed lamina was assessed using computed tomography (CT) at 6 months after surgery.
Results
Spinous process-slitting hemilaminoplasty was performed for intradural extramedullary tumors in eight patients and for ossification of the ligament flavum in one patient. Because we were able to visualize the margin of the ipsilateral and contralateral dura, we were able to secure space for removal of the lesion and closure of the dura. None of the cases showed spinal deformity or other complications. Bone fusion and maintenance of the spinal canal were found to be perfect on CT scans.
Conclusion
The spinous process-splitting hemilaminoplasty technique presented here was successful in creating sufficient space to remove intradural and posterior epidural lesions and to close the dura. Furthermore, we were able to maintain the physiological barrier and integrity after surgery because the posterior musculature and bone structures were restored.
INTRODUCTION
Laminectomy without reconstruction has been the standard surgical treatment method for intradural and posterior epidural spinal lesions7). Although this surgical technique provides an adequate operative field, it may lead to hematoma in the spinal canal, invasion of scar tissue after surgery, or postoperative instability and scoliosis45). As microscopic surgical techniques have evolved, new surgical methods such as laminoplasty with the use of a T-saw for reconstruction36) or laminoplasty with a 90° or 60° change in the lamina with en bloc removal14) have been introduced. Some authors have reported that certain spinal cord tumors can be removed through hemilaminectomy and a minimally invasive technique that involves the use of a tubular retractor system, which enables maximum preservation of the posterior elements210). Here, we report a spinous process-splitting hemilaminoplasty technique that maintains the shape and integrity of the posterior elements and retains a barrier that prevents hematoma in the spinal canal and scar tissue damage.
MATERIALS AND METHODS
Patients
Between 2011 and 2014, spinous process-splitting hemilaminoplasty was performed in nine patients. Physical examinations, MRI with gadolinium, CT, and routine radiography with dynamic imaging of the intradural and posterior epidural lesions were performed prior to surgery.
Surgical procedures
Spinous process-splitting hemilaminoplasty involves a posterior midline incision in the prone position. Unilateral paravertebral muscle dissection reveals the lamina and a part of the medial facet joint.
Surgical procedures are summarized in Fig. 1. A hole is created in the spinous process, lamina, and medial articular facet with a match head burr (1.7 mm) drill to achieve laminoplasty to replace the bone that was removed en bloc into the exact pre-surgery position.

A : Intradural spinal lesion (red). B : hemilaminectomy after splitting of the spinous process (using ossillating saw or T-saw) and laminofacet jounction (using match head burr). C : Contralateral spinolamina junction undercutting and exposure contralateral dural margin. D : Removal intraldural lesions and performed hemilaminoplasty.
The splitting of the spinous process can be performed by 1) cutting to a depth determined on the basis of measurements on computed tomography (CT) scans with an oscillating saw in the midline where the epidural space is wide or 2) splitting with the T-saw by partially removing the surrounding ligament flavum to facilitate use of the T-saw, revealing the dura and enabling passage of the T-saw stylet. In the cases reported here, we used both methods and found that the T-saw method resulted in less bone loss due to the cutting. Using a fine burr, we linearly drilled the laminofacet junction into the spinal canal in order to perform the hemilaminectomy.
This approach secures a wider operating field than previous hemilaminectomy procedures, as the surgeon is able to see as much as half of the spinous process. Furthermore, undercutting of the opposite side of the spinolamina junction enables the surgeon to see the dura margin on the contralateral side, providing enough space to treat intradural and posterior epidural lesions. Finally, the 1-cm operating field is wide enough to place dura sutures.
After removing the spinal lesion, we performed suture for the spinous process and the laminofacet junction, which enabled laminoplasty with similar pre-surgery integrity. Of note, placing the sutures on the split spinous process results in a stable suture on the laminofacet junction without subsequent movement. Non-absorbable suture threads were used to stabilize the surgical area and to prevent the formation of artifacts. This is beneficial given that imaging techniques such as magnetic resonance imaging (MRI) are used for follow-up inspection. Surgical outcomes were evaluated on the basis of postoperative symptom states and complications, operation time, and estimated blood loss (EBL) (Table 2).
RESULTS
The age of the patients ranged from 28 to 76 years (mean, 60.2 years). Four patients had radiculopathy and five exhibited motor weakness. Eight had an intradural extramedullary spinal tumor and one had ossification of the ligament flavum. The surgical sites were cervical in six patients, thoracic in one, and lumbar in two (Table 1). Spinous process-splitting hemilaminoplasty was performed on levels 1-3 with a mean operation time of 237.8±47.0 min (range, 160-315 min) and a mean EBL of 116.7±61.2 mL (range, 50-200 mL). Post-surgical diagnoses were schwannoma in five patients, meningioma in two, neurofibroma in one, and ossification of the ligament flavum in one. We performed total resection in the schwannoma, neurofibroma, and ossified ligament flavum cases, and Simpson grade II resection in the patients with meningioma (Table 2).

Characteristics of the patients who underwent spinous process-splitting hemilaminoplasty for intradural extramedullary and cauda equine spinal tumors
The postoperative follow-up period was 6 to 31 months, and all patients were able to walk 1 day after surgery. All patients recovered without neurologic deficits or pain aggravation. CT scans were obtained for each patient 6 months after surgery, and the findings confirmed that laminoplasty was achieved on the bone fusion without displacement.
ILLUSTRATIVE CASE PRESENTATIONS
Case 1
A 57-year-old female patient had pain and numbness in her left arm for 3 months. Cervical MRI revealed an intradural extramedullary spinal tumor leaning toward the left at C3-4-5 (Fig. 2A, B). We split the spinous process on the left side of C3-4-5, drilled the facet lamina junction, and performed en bloc hemilaminectomy. After removing the tumor, we placed the detached bone fragments in their original position using non-absorbable suture threads (Fig. 2C). The pathological diagnosis was schwannoma. CT performed 6 months after surgery revealed that bone fusion had been achieved (Fig. 2D).
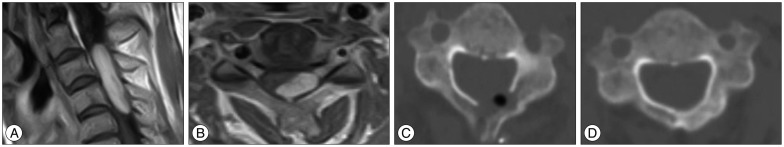
Case 1. A 57-year-old woman with an intradural extramedullary schwannoma at C3-4-5. Preoperative gadolinium-enhanced T1-weighted magnetic resonance imaging, where the sagittal and axial images reveal a left-sided intradural extramedullary lesion (A and B). Computed tomography scans obtained immediately after surgery (C) and 6 months after spinous process-splitting hemilaminoplasty (C3-4-5) (D), suggesting complete bone fusion.
Case 5
A 55-year-old male patient had progressive radiating pain in the right leg. MRI revealed an intradural spinal tumor at L1-2 (Fig. 3A, B), and a spinous-splitting hemilaminectomy was performed. To achieve optimal vision, undercutting of the contralateral spinous process was performed, which enabled the surgeon to see both sides of the dura to the same extent (Fig. 4). After the tumor was removed, laminoplasty was performed (Fig. 3C).

Case 5. A 55-year-old man with a schwannoma. A : Preoperative T2-weighted axial magnetic resonance image. B : Preoperative axial computed tomography image. The tumor was completely removed via spinous process-splitting hemilaminoplasty at L1. C : Computed tomography scans obtained immediately after the laminoplasty.
Case 7
A 62-year-old female patient had progressive pain and numbness in the right arm 3 months prior to her hospital visit. MRI revealed an intradural extramedullary spinal tumor on the right side of C5-6 (Fig. 5A, B). Spinous process-splitting hemilaminectomy was performed on the right side of C5-6, and the tumor was removed. Laminoplasty was performed in the same position, using non-absorbable threads (Fig. 5C).
DISCUSSION
Although laminectomy has previously been used to treat intradural lesions, the surgical outcomes have often been compromised by hematoma, scar tissue invasion, and/or postoperative instability. With the development of microsurgical techniques, various alternative methods have been introduced1249). Alternative methods include rotation laminoplasty, hemilaminectomy, and en bloc laminoplasty.
Asazuma et al.1) performed 90 degree-rotation laminoplasty on spinal cord and cauda equina tumors in seven patients and reconstructed the vertebral arch. The authors reported satisfactory operative exposure, a maintained range of motion, and an improved Japanese Orthopedic Association score. Hida et al.4) introduced a 60 degree-rotation laminoplasty with titanium mini-plates. Of the eight patients who underwent surgery, six had an intradural tumor as well as ossification of the ligament flava and spontaneous spinal cord herniation.
These methods involve rotating the lamina because of bone loss due to cutting. This can only be conducted on the thoracic and lumbar spine where the spinous process is long, but not on the cervical spine where the spinous process is short. Therefore, these techniques can only be used to treat thoracic and lumbar lesions. Furthermore, because bone fusion does not mirror the original state, this technique cannot be considered true reconstructive laminoplasty. In contrast, the method that we introduced here involves minimizing bone loss by cutting the spinous process with a T-saw or oscillating saw to obtain a wide epidural space, enabling preservation of the pre-surgical appearance. This technique is not only applicable to the thoracic and lumbar spine regions, but also to cervical lesions. In addition, because it does not involve the use of titanium mini-plates, there are no artifacts, and therefore, imaging evaluations are more accurate, especially in cases of tumors that could be recurrent.
Hemilaminectomy has been introduced as a surgical treatment for intradural lesions using a unilateral approach28). It preserves the contralateral muscles and ligaments, which prevents secondary instability and scoliosis after laminectomy. However, in hemilaminectomy, the lamina is removed via a unilateral approach, and thus, the operative field is restricted. In contrast, our technique involves splitting the spinous process, thereby allowing the midline of the dura to be seen (Fig. 3). Undercutting of the spinous process enables the surgeon to see around the midline of the dura and dura margins, and therefore, both sides become visible.
Hara et al.3) performed en bloc laminoplasty on 16 patients using a T-saw, and similar to the results obtained using our technique, the bone shape remained similar before and after surgery. The authors reported that by cutting the spinous process around the surgical level and placing the sutures, they were able to preserve posterior supporting elements such as the supraspinous and infraspinous ligaments, preventing kyphotic deformity after laminoplasty. However, our method involves a smaller incision because the spinous process around the surgical area is not removed. Moreover, the midline and contralateral posterior elements remain intact, and thus, the physiological integrity of the spinous ligament is maintained while postoperative kyphotic deformity is prevented. It should also be noted that splitting the spinous process where there is a large amount of cancellous bone, rather than the laminofacet junction, results in better bone fusion.
Spinous process-splitting hemilaminoplasty and other surgical methods were compared with respect to operative time and blood loss12348). The operation time for 60-degree rotation laminoplasty3) was 233.75±63.46 min (170-380) and blood loss was 218.75±176.33 mL (45-600), while for 90-degree rotation laminoplasty1), the operation time was 338.29±144.35 min (205-445) and blood loss was 578.43±176.33 mL (73-2038). For heminlaminectomy8), operative time was 246.67±61.54 min (180-320) and blood loss during surgery was 55.83±12.01 mL. For en-bloc laminoplasty3), surgery time was 350.63±81.20 min (242-485) and blood loss was 589.31±282.89 mL (90-1209). These results are similar to those obtained using our surgical technique. And the minimal operative time and bleeding on our surgical method were similar for other surgical methods.
The majority of spinal tumors are either intradural extramedullary (40%) or extradural (55%) tumors, and the intradural lesions often lean toward one side8). In our cases, during same period, we treated a total of 11 cases of spinal cord tumors. Except for two patients with intradural intramedullary tumors and one patient with previous posterior spine surgery, spinous process-splitting hemilaminoplasty was performed in all cases where conventional techniques were applicable. Complete tumor removal was achieved in all cases.
Our novel surgical technique has advantages over previous laminectomy, laminoplasty, and hemilaminectomy techniques. Primarily, this new procedure enables adequate visualization during surgery and prevents scar tissue invasion and hematoma. Moreover, it does not cause injury to the muscles and ligaments on the contralateral side of the lesion, and thus prevents postoperative instability and subluxation. Finally, it should be noted that no imaging artifacts are produced as a result of the surgery, and therefore, imaging evaluations related to tumor recurrence are more accurate.
CONCLUSION
Spinous process-splitting hemilaminoplasty for intradural and posterior epidural lesions preserves the contralateral posterior elements and maintains stability compared to previous reconstructive laminoplasty techniques. Visualization is also improved compared to previous hemilaminectomy techniques, and laminoplasty following this procedure helps maintain physiological integrity.