Central Sarcopenia, Frailty and Comorbidity as Predictor of Surgical Outcome in Elderly Patients with Degenerative Spine Disease
Article information
Abstract
Objective
People are living longer and the elderly population continues to increase. The incidence of degenerative spinal diseases (DSDs) in the elderly population is quite high. Therefore, we are facing more cases of DSD and offering more surgical solutions in geriatric patients. Understanding the significance and association of frailty and central sarcopenia as risk factors for spinal surgery in elderly patients will be helpful in improving surgical outcomes. We conducted a retrospective cohort analysis of prospectively collected data to assess the impact of preoperative central sarcopenia, frailty, and comorbidity on surgical outcome in elderly patients with DSD.
Methods
We conducted a retrospective analysis of patients who underwent elective spinal surgery performed from January 1, 2019 to September 30, 2020 at our hospital. We included patients aged 65 and over who underwent surgery on the thoracic or lumbar spine and were diagnosed as DSD. Central sarcopenia was measured by the 50th percentile of psoas : L4 vertebral index (PLVI) using the cross-sectional area of the psoas muscle. We used the Korean version of the fatigue, resistance, ambulation, illnesses, and loss of weight (K-FRAIL) scale to measure frailty. Comorbidity was confirmed and scored using the Charlson Comorbidity Index (CCI). As a tool for measuring surgical outcome, we used the Clavien-Dindo (CD) classification for postoperative complications and the length of stay (LOS).
Results
This study included 85 patients (35 males and 50 females). The mean age was 74.05±6.47 years. Using the K-FRAIL scale, four patients were scored as robust, 44 patients were pre-frail and 37 patients were frail. The mean PLVI was 0.61±0.19. According to the CD classification, 50 patients were classified as grade 1, 19 as grade 2, and four as grade 4. The mean LOS was 12.35±8.17 days. Multivariate stepwise regression analysis showed that postoperative complication was significantly associated with surgical invasiveness and K-FRAIL scale. LOS was significantly associated with surgical invasiveness and CCI. K-FRAIL scale showed a significant correlation with CCI and PLVI.
Conclusion
The present study demonstrates that frailty, comorbidity, and surgical invasiveness are important risk factors for postoperative complications and LOS in elderly patients with DSD. Preoperative recognition of these factors may be useful for perioperative optimization, risk stratification, and patient counseling.
INTRODUCTION
People are living longer and the elderly population continues to increase worldwide. The population is expected to age rapidly in most countries, including Korea. Korea became an aging society (elderly population ≥7% of the total population) in 2000. In 2018, Korea became an aged society (defined as an elderly population ≥14% of the total population), and by 2026 Korea will be a super-aged society (elderly population ≥20% of the total population) [37]. The incidence of degenerative spinal disease (DSD) in the elderly population is quite high [36,38]. Therefore, we are facing more patients with DSD and initiating surgery in increasing numbers of geriatric patients [20].
The incidence of perioperative complications is higher in elderly patients than in younger patients, resulting in higher morbidity and mortality in this patient population [19]. Although age is associated with an increased risk of surgical complications, this does not mean that spinal surgery cannot be safely performed due to advanced age [36]. However, some surgeons are reluctant to perform surgery in elderly patients because of the high risk of perioperative complications due to the patient’s old age. Thus, clarifying risk of surgery in elderly patients is an important factor in decision-making and the preoperative optimization of patients [22]. Assessing the risk of surgery of an individual patient can be difficult, especially in the elderly, due to discrepancies between an individual’s physiological age and chronological age [17].
Frailty and sarcopenia have been attracting attention as factors for evaluation of physiological age in recent studies of risk factors for surgery in elderly patients [16,26]. Frailty is defined as a medical syndrome characterized by a decline in strength and endurance and reduced physiologic function that increase the vulnerability for increased dependency [31]. Frailty is a universal risk factor for recurrent falls, delirium, institutionalization, and poor postoperative outcome [26]. Recently, there has been interest in the gerontology field in quantifying the increased risk of perioperative complications from frailty [1,22,35,38]. The physical phenotype of frailty shares the characteristic of decrease of muscle strength, similar to sarcopenia [4]. The term “sarcopenia” is used to indicate progressive reduced muscle mass, reduced muscle strength, and reduced function that affects older adults [10,28]. Recent studies have reported that central sarcopenia, as measured by psoas cross-sectional area, has been associated with adverse events after cardiovascular surgery [2,18,24,30,39]. However, the impact of central sarcopenia in spine surgery for elderly patients has not yet been well characterized. Understanding the significance and association of frailty and central sarcopenia as risk factors for spinal surgery in elderly patients will be helpful in improving surgical outcomes.
We conducted a retrospective cohort analysis of prospectively collected data to assess the impact of preoperative central sarcopenia, frailty, and comorbidity on surgical outcome in elderly patients with DSD.
MATERIALS AND METHODS
Study design
This study protocol was approved by the Institutional Review Board (IRB) and Ethics Committee of Soonchunhyang University Seoul Hospital (IRB number : 2020-11-030). We conducted a retrospective analysis on patients who underwent elective spinal surgery performed from January 1, 2019 to September 30, 2020 at Soonchunhyang University Seoul Hospital. We included patients aged 65 and over who underwent surgery on the thoracic or lumbar spine and who were diagnosed as DSD. We excluded cases of infection, trauma, reoperation, tumor, and infection. Patient characteristics, such as age, sex, body mass index (BMI), and body surface area (BSA), were collected using electronic medical records.
Central sarcopenia
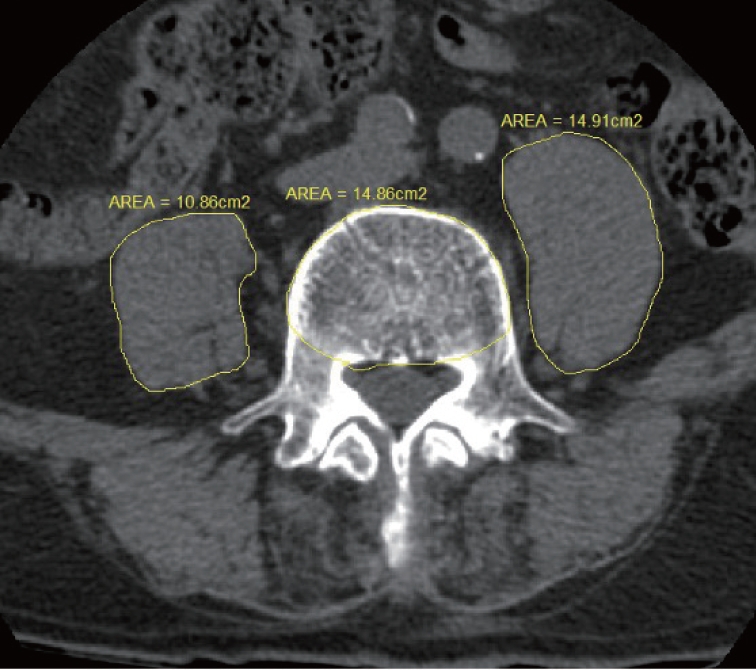
There are two main methods for measuring sarcopenia : measuring muscle performance or imaging muscle mass [10]. We measured central sarcopenia by measuring the cross-sectional area of the psoas muscle [15]. Using preoperative computed tomography (CT) scan, the cross-sectional areas of the left and right psoas muscles were calculated at the level of the L4 pedicle. To reduce error, these measurements was calculated by two researchers and averaged. To normalize for body habitus, the cross-sectional area of the L4 vertebral body was also recorded at this level (Fig. 1). We calculated the 50th percentile of psoas : L4 vertebral index (PLVI) using the following formula [15] : [right PCSA (mm2) + left PCSA (mm2) / 2] / L4 vertebral CSA (mm2).
Frailty
Many studies worldwide have investigated frailty, and various methods for evaluating frailty including the frailty index have been reported [26]. In this study, we used the Korean version of the fatigue, resistance, ambulation, illnesses, and loss of weight (K-FRAIL) scale to evaluate frailty. The K-FRAIL scale, based on the original FRAIL scale published by Morley et al. [29], was translated into Korean [21] and is a simplified index suitable for the Korean medical environment. The K-FRAIL scale is composed of a simple 5-item questionnaire (addressing fatigue, resistance, ambulation, illness, loss of weight) with no requisite for physical examination and is an easy screening method for frailty status. In this questionnaire, a score of 0 is considered as robust, 1 to 2 as pre-frail, and 3 to 5 as frail (Supplementary Fig. 1 and Supplementary Table 1).
Comorbidity
For the analysis of postoperative outcome, comorbidity was confirmed and scored as an evaluation index of the patient’s condition before surgery. The Charlson Comorbidity Index (CCI) [32] quantifies comorbidity with reference to medical records and has been used in many clinical practices as a measure of predicting patient prognosis and outcome. The CCI was calculated from the International Classification of Diseases-10 comorbidity codes, using 17 comorbidities (Supplementary Table 2). Each comorbidity is assigned a score (weight) when computing the weighted CCI. Diabetes with complications, hemiplegia, renal disease, and cancer are assigned a score of 2; metastatic cancer and severe liver disease are assigned a score of 3; human immunodeficiency virus is assigned a score of 6; and the remaining comorbidities are assigned a score of 1 [32].
Surgical invasiveness
The type of surgery was analyzed using the surgical invasiveness index. The surgical invasiveness index is a previously validated tool that can describe the number of vertebral levels decompressed, arthrodesed, or instrumented as well as the type of approach [9]. This index ranges from 0 to 48 points, and a higher the score indicates a greater surgical invasiveness. The index is the sum of six weighted surgical components : anterior decompression, anterior fusion, anterior instrumentation, posterior decompression, posterior fusion, and posterior instrumentation. The weights for each component represent the number of vertebral levels at which each is performed. The operation time was analyzed in minutes.
Surgical outcome
To measure surgical outcome, we used the presence or absence of postoperative complications and the length of stay (LOS). For postoperative complications, the Clavien-Dindo (CD) classification was used [3]. This classification is graded from 1 to 5 according to increasing severity and the type of intervention required to treat the complication (Supplementary Table 3) [3]. In this study, grades II, III, IV, and V were considered as the presence of postoperative complications. LOS was calculated from the date of surgery to discharge, not the number of days from admission to discharge.
Statistical analysis
Postoperative complication and LOS were set as dependent variables, and patient variables such as sex, age, CCI, BMI, BSA, K-FRAIL scale, operation time, and surgical invasiveness index were set as independent variables. First, univariate analysis was performed; several variables were selected and multivariate linear regression analysis was performed to analyze the correlation between the variables. In addition, to investigate the correlation between the K-FRAIL scale and central sarcopenia, a correlation analysis between the K-FRAIL scale, PLVI, CCI was performed to calculate the significance level and correlation coefficient. All statistical analyses were performed using SPSS version 26 (IBM, Armonk, NY, USA) and statistical significance was defined as a p-value <0.01.
RESULTS
Patient demographics
A total of 128 patients were initially selected; after excluding 43 patients based on exclusion criteria as described in the MATERIALS AND METHODS, this study included 85 patients. The patient group included 35 males (41.2%) and 50 females (58.8%); the mean age was 74.05±6.47 years. The average BMI and BSA were 25.12±2.97 kg/m2 and 1.64±0.19 m2, respectively. Regarding the K-FRAIL scale, four patients (4.7%) were scored as robust, 44 patients (51.8%) were pre-frail and 37 patients (43.5%) were frail. The mean PLVI was 0.61±0.19. Surgical diagnoses included spinal stenosis (51 patients), herniation of intervertebral disc (16 patients), spondylolisthesis (10 patients), and other (eight patients). Regarding surgical invasiveness, 16 patients (18.8%) were scored 8 points or more. The mean operative time was 154.84±85.54 hours. According to the CD classification, 50 patients were classified as grade 1, 19 as grade 2, and four as grade 4. The mean LOS was 12.35±8.17 days (Table 1).
Postoperative complications
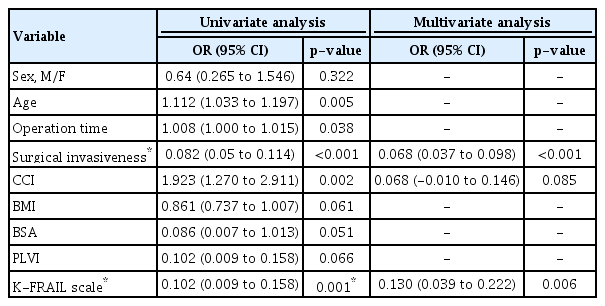
Postoperative complications occurred in 35 patients (41.2%). The most common complications were poor oral intake (16 patients, 18.8%), voiding difficulty (14 patients, 16.5%), urinary tract infection (four patients, 4.7%), pneumonia (three patients, 3.5%), and delirium (three patients, 3.5%) (Table 2). Univariate analysis showed that age, surgical invasiveness, CCI, and K-FRAIL scale were statistically significant : age (odds ratio [OR], 1.112; 95% confidence interval [CI], 1.033–1.197; p=0.05), surgical invasiveness (OR, 1.361; 95% CI, 0.841–1.881; p<0.001), CCI (OR, 1.923; 95% CI, 1.270–2.911; p=0.002), and K-FRAIL scale (OR, 0.102; 95% CI, 0.009–0.158; p=0.001). Multivariate stepwise regression analysis showed that postoperative complication was significantly associated with surgical invasiveness (p<0.001) and K-FRAIL scale (p=0.006) (Table 3).
LOS
The mean LOS was 12.35±8.17 days. Univariate analysis showed that age, operation time, surgical invasiveness, CCI, and K-FRAIL scale were statistically significant : age (OR, 0.416; 95% CI, 0.156–0.676; p=0.002), operation time (OR, 0.026; 95% CI, 0.06–0.046; p=0.011), surgical invasiveness (OR, 1.361; 95% CI, 0.841–1.881; p<0.001), CCI (OR, 3.150; 95% CI, 1.878–4.423; p<0.001), and K-FRAIL scale (OR, 2.612; 95% CI, 0.983–4.241; p=0.002). Multivariate stepwise regression analysis was performed, and LOS was associated with surgical invasiveness (OR, 1.088; 95% CI, 0.606–1.571; p<0.001) and CCI (OR, 2.204; 95% CI, 0.974–3.435; p=0.001) (Table 4).
Central sarcopenia and frailty
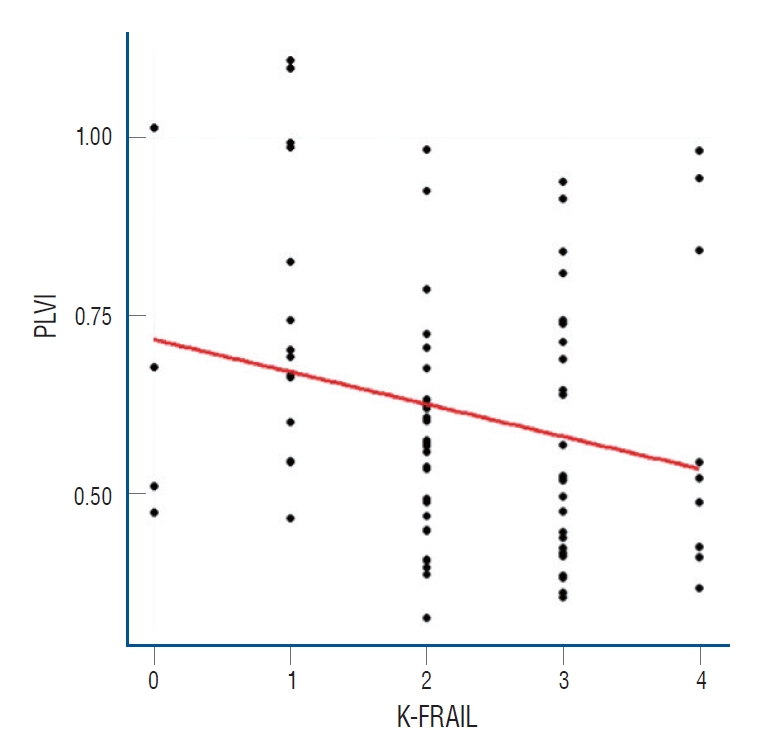
Pearson correlation analysis was performed between the K-FRAIL scale and PLVI. The Pearson correlation coefficient was -0.242, and the p-value was 0.026. This showed a significant negative correlation (Fig. 2).
DISCUSSION
There is a general assumption that the outcomes of spine surgery in the geriatric population are worse than in the young and middle-aged populations. However, this has not been demonstrated in the literature [1,38]. The most important reason for this may be due to widely varying discrepancies between an individual patient’s physiological age and chronological age [17]. Therefore, frailty and sarcopenia have been attracting attention as factors for evaluating the physiological state of elderly patients.
Frailty is characterized by physiological age-related decreases in energy, cognitive function, physical health, and other deficits that collectively make the patient more vulnerable to stressors, resulting in adverse outcomes [4,6,17]. Consensus on how best to define and measure frailty remains elusive [1,2,5]. Frailty has been measured using different methods in the literature. Two major models of defining frailty are the frailty phenotype and the deficit accumulation model, also known as the frailty index [26,35]. Segal et al. [33] found that higher scores from the 5-item modified Frailty Index were associated with an increased rate of complications and readmission in patients who underwent kyphoplasty. A modified Frailty Index based on the Canadian Study of Health and Aging-Frailty Index, which comprises 11 items, has also been proven effective in various surgical populations [23,26]. However, this index does not adequately reflect physical phenotype. An ideal tool for assessing frailty would be quick and cost-effective, incorporating relevant criteria that are easily disseminated for clinical applications as well as research [1]. The K-FRAIL scale is correlated with the frailty index and is a simple tool to screen for frailty in a clinical setting [21]. The questionnaire can be relatively easily and quickly administered for estimating the physical phenotype. To the best of our knowledge, this is the first study using the K-FRAIL scale screening method for spine surgery of the elderly population.
Previous evidence has indicated that higher levels of frailty correlate independently with mortality, postoperative complications, prolonged hospital LOS, and discharge to a rehabilitation facility in surgery of elderly populations [1,17,20,22,23,26,35,38]. Our study showed that postoperative complications were significantly associated with frailty measured by the K-FRAIL scale. Spinal surgeons need to recognize the correlation between frailty and preoperative risk in the elderly population. The ability of a frailty index to estimate postoperative complications depends on the index used to measure frailty. Therefore, tools to assess frailty are even more crucial. The K-FRAIL scale is recommended as a screening test for the evaluation of frailty in spinal surgery for elderly patients due to its simple, convenient, and measurement of physical phenotype.
The term “sarcopenia” is used to indicate progressive low muscle mass, low muscle strength, and low function that affect older adults [11,16,28]. Sarcopenia is one element of frailty syndrome that is unaffected by acute changes in clinical status or patient effort [2]. Reduced grip strength of sarcopenia is common criteria in physical frailty [11]. The cause of sarcopenia is generally thought to be multifactorial [11,28]. Sarcopenia is associated with increased adverse outcomes including a loss of independence, frailty, poor quality of life, increased healthcare costs, and mortality [11,14,28]. Although the prevalence of sarcopenia may vary in the same cohort due to different diagnostic criteria, the prevalence of sarcopenia globally will continue to increase because of the overall increase in aging populations [10,37]. Diagnosis of sarcopenia requires measurement of a combination of muscle mass, muscle strength, and physical performance [10,11]. The most effective assessment for diagnosis involves the use of dual energy X-ray absorptiometry, bioelectrical impedance analysis, CT, and magnetic resonance imaging (MRI), and these can also have a role in some setting associated with other diseases [11,14]. CT and MRI are mostly used in diagnosis and when needed for the follow-up of changed conditions in patients with DSD. Therefore, evaluation of sarcopenia using CT and MRI can be very useful for patients with spinal diseases.
Central sarcopenia through measuring psoas muscle has recently been studied as a predictor of outcome in elderly medical patients [24]. Psoas muscle area (PMA) can be measured on CT scans and has been shown to correlate well with whole body muscle mass [18,34]. In cardiac transplant patients, lower PMA is also associated with increased in-hospital mortality or major morbidity and worse long-term survival [2,6,39]. The psoas muscle impacts functional recovery after major surgery. A validated method for quantifying sarcopenia is measurement of the PMA [2,18,24,39]. PMA can be quantified using cross-sectional CT scans with good inter-observer correlation, and PMA correlates with sarcopenia [2,24]. The PLVI using CT scan in our study has the advantage that it can be easily measured in all patients with spinal disease [15]. However, whether PMA is the most sensitive or specific indicator for frailty has not yet been determined [2].
In our study, central sarcopenia assessed using PLVI on CT was statistically associated with the K-FRAIL scale but was not strongly associated with postoperative complication and LOS. Our results suggest that PLVI is associated with frailty but is not a significant risk factor for postoperative outcomes. Whether PLVI can accurately reflect sarcopenia and whether sarcopenia is less associated with the outcome of spinal surgery should be investigated, and further research is required to address these questions.
Comorbidity is an established risk factor for complications during spine surgery [27,38]. In 1987, Charlson et al. [8] developed an age-based CCI score, but subsequent studies showed that age and CCI should be examined separately in multivariable predictor analyses of surgical outcomes, especially with large sample sizes [7]. In our study, a higher CCI appeared to associate with increased LOS after surgery. In spinal surgery of elderly patients, comorbidities should be concerned before planning surgery.
Fusion surgery leads to more complications than decompression surgery due to increased blood loss, longer operation time, and higher infection rate [20,22]. Dagistan et al. [12] concluded that increased operative times and instrumentation and fusion procedures were strongly associated with an increased risk of developing complications. Kobayashi et al. [25] concluded that reducing surgical invasiveness is important in treatment of very elderly patients. Deyo et al. [13] showed that as the complexity of surgery increases, the risk of life-threatening complications also increases. The surgical invasiveness index used in our study is a previously validated instrument that accounts for the number of vertebral levels decompressed, arthrodesed, or instrumented as well as the surgical approach [9]. Our study demonstrated that as the surgical invasiveness increased, postoperative complications and LOS increased. These findings further validate the importance of surgical invasiveness when performing spine surgery in elderly patients.
Historically, surgeons have relied on clinical experience to determine patients for surgery, but risk assessment and stratification also ascertain the ability of patients to tolerate surgery. Accurate and timely risk stratification before surgery is crucial to ensure successful outcomes, especially among older and higher risk populations [1,38]. A risk assessment must be made for each patient in a specific surgery. This requires a systematic and integrated approach and evaluation of risk factors related to surgery and patients. In our study, we examined the relationship of complications by CD classification using the K-FRAIL scale, PMA measurements and the surgical invasiveness index, which can be conveniently used in the outpatient clinic. The findings of our study have important implications in risk stratification, perioperative planning, and the counseling of elderly patients with DSD. Assessment of risk factors should be implemented in the pre-surgical period, including during pre-rehabilitation, nutritional supplementation, medication adjustment, and preoperative medication [38].
Limitations
This study has several limitations. First, there were only a small number of patients in the study. Second, there is a bias associated with data collection through the national insurance system. In our study, the result for no correlation between the LOS and the K-FRAIL scale is highly likely to have been distorted by an insurance system in which the LOS is limited by the type of surgery and disease. Finally, we did not assess the association between PLVI using CT scan and sarcopenia. This fact limits the interpretation of the relationship between PVLI and surgical outcomes. This requires further research.
CONCLUSION
The present study demonstrates that frailty, comorbidity, and surgical invasiveness are important risk factors for postoperative complication and LOS in elderly patients with DSD. Preoperative recognition of these factors may be useful for perioperative optimization, risk stratification, and patient counseling. Central sarcopenia assessed by PLVI using CT scan was associated with the K-FRAIL scale. Future research should further assess the significance of central sarcopenia in this scale as a risk factor for surgical outcome.
Notes
CONFLICTS OF INTEREST
Sukh Que Park has been editorial board of JKNS since November 2018. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : HKP
Data curation : DUK, GHL
Formal analysis : DUK, HKP, GHL
Funding acquisition : JCC
Methodology : HRP
Project administration : SJC
Visualization : SQP
Writing - original draft : DUK
Writing - review & editing : HKP
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2021.0074.
The Korean version of the fatigue, resistance, ambulation, illnesses, and loss of weight (K-FRAIL) scale
Charlson Comorbidity Index
Clavien-Dindo classication
The Korean version of the fatigue, resistance, ambulation, illnesses, and loss of weight (K-FRAIL) scale.