Comparison of Fusion Rate between Demineralized Bone Matrix versus Autograft in Lumbar Fusion : Meta-Analysis
Article information
Abstract
The demineralized bone matrix (DBM) as the bone graft material to increase the fusion rate was widely used in spinal fusion. The current study aimed to compare the fusion rate of DBM to the fusion rate of autograft in lumbar spine fusion via meta-analysis of published literature. After systematic search, comparative studies were selected according to eligibility criteria. Checklist (risk of bias assessment tool for non-randomized study) was used to evaluate the risk of bias of the included nonrandomized controlled studies. The corresponding 95% confidence interval (95% CI) were calculated. We also used subgroup analysis to analyze the fusion rate of posterolateral lumbar fusion and lumbar interbody fusion. Eight studies were finally included in this meta-analysis. These eight studies included 581 patients. Among them, 337 patients underwent spinal fusion surgery using DBM (DBM group) and 204 patients underwent spinal fusion surgery with mainly autologous bone and without using DBM (control group). There was no significant differences of fusion rate between the two groups in posterolateral fusion analysis (risk ratio [RR], 1.03; 95% CI, 0.90–1.17; p=0.66) and interbody fusion analysis (RR, 1.13; 95% CI, 0.91–1.39; p=0.27). Based on the available evidence, the use of DBM with autograft in posterolateral lumbar spine fusion and lumbar interbody fusion showed a slightly higher fusion rate than that of autograft alone; however, there was no statistically different between two groups.
INTRODUCTION
Bone fusion is a very important process in spinal fusion surgery. Failure to achieve bone fusion after surgery leads to several complications. Pseudarthrosis, the result of failed spinal fusion, can cause implant migration or breakage and pedicle screw loosening. With further progression of pseudarthrosis, spinal instability can be initiated and the clinical outcome worsens [16,33].
To prevent pseudarthrosis in spinal fusion surgery, proper use of implant and preparation of bone fusion bed are two important aspects. However, the selection of good bone graft material is also a critical factor. Autologous iliac crest bone graft (ICBG) is the standard bone graft material in spinal fusion surgery [10,11]. However, the amount of autologous ICBG is limited, and there are several complications due to ICBG usage, such as hematoma, infection, and pain [3,31]. Therefore, surgeons require an alternative bone graft material with osteoinductive and osteoconductive ability equivalent to that of ICBG [15].
Demineralized bone matrix (DBM), prepared from allograft bone by demineralization techniques, theoretically has bone morphogenetic protein (BMP) and osteoinductive ability [2]. According to previous studies, the effect of DBM in spinal fusion surgery is still controversial [6,32]. We therefore conducted this meta-analysis to further evaluate the fusion rate of group using DBM compared to that of another group using autograft bone in lumbar spinal fusion surgery.
MATERIALS AND METHODS
Study selection
To identify the relevant studies, we searched the following databases using controlled vocabulary and free-text words described in Supplementary Material : Pubmed (MEDLINE), EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the Web of Science, and SCOPUS. This study is based on the Cochrane Review Methods, and reporting was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We aimed to identify all relevant studies regardless of language, publication type (article, poster, conference article, etc.), journal and date. This search was updated in March 2019 and includes reference list of the studies and review articles identified. There were no restrictions on start dates for articles found.
Eligibility criteria
We selected studies based on the following criteria : 1) studies including patients who underwent lumbar spinal fusion surgery with screw fixation due to degenerative changes in the spine; 2) studies comparing bone fusion rates between group using autologous bone graft and another group using DBM in patients who underwent lumbar spinal fusion surgery; and 3) studies evaluating bone fusion rate using radiography and computed tomography (CT). Animal studies and non-comparative studies were excluded.
Data collection
Two reviewers independently recorded data from each study. Any disagreement between the two reviewers about data extraction was settled by the opinion of a third reviewer. The following data were extracted from the studies: study design, patient demographics, performed interventions, radiographic and clinical outcomes, statistical methods, and study results.
Fusion evaluation
The meta-analysis included only papers describing the fusion evaluation method. For radiographic evaluation of fusion, the evaluation should be carried out using previously published criteria or meet similar comparative criteria. Criteria included having the continuous bony bridging in CT scans or X-rays and/or minimal lack of movement in dynamic plain film.
Assessment of methodological quality
The risk of bias and the methodological quality were assessed as described in the Cochrane Handbook for Systematic Reviews of Interventions [13]. The risk of bias for non-randomized experimental studies was assessed using the Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) for quality assessment. We evaluated the degree of effectiveness and quality of evidence for each outcome. Two investigators independently assessed the methodological quality of each study. Any disagreement between the two reviewers was resolved by consensus or by consultation with a third investigator. Publication bias was not assessed due to the small number of enrolled studies.
Statistical analysis
The meta-analysis was conducted using Review Manager (RevMan) version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The randomeffects model was suitable for our study and was used to analyze fusion data. To evaluate fusion rate, we calculated the risk ratio (RR) in the included studies.
The heterogeneity among the studies was assessed using I2 statistic, with I2 values of 25%, 50%, and 75% considered to be low, moderate, and high, respectively. Cochran’s Q statistic (chi-square test) was also used to assess the heterogeneity, with a p value <0.10 defining significant degree of heterogeneity.
RESULTS
Identification of studies
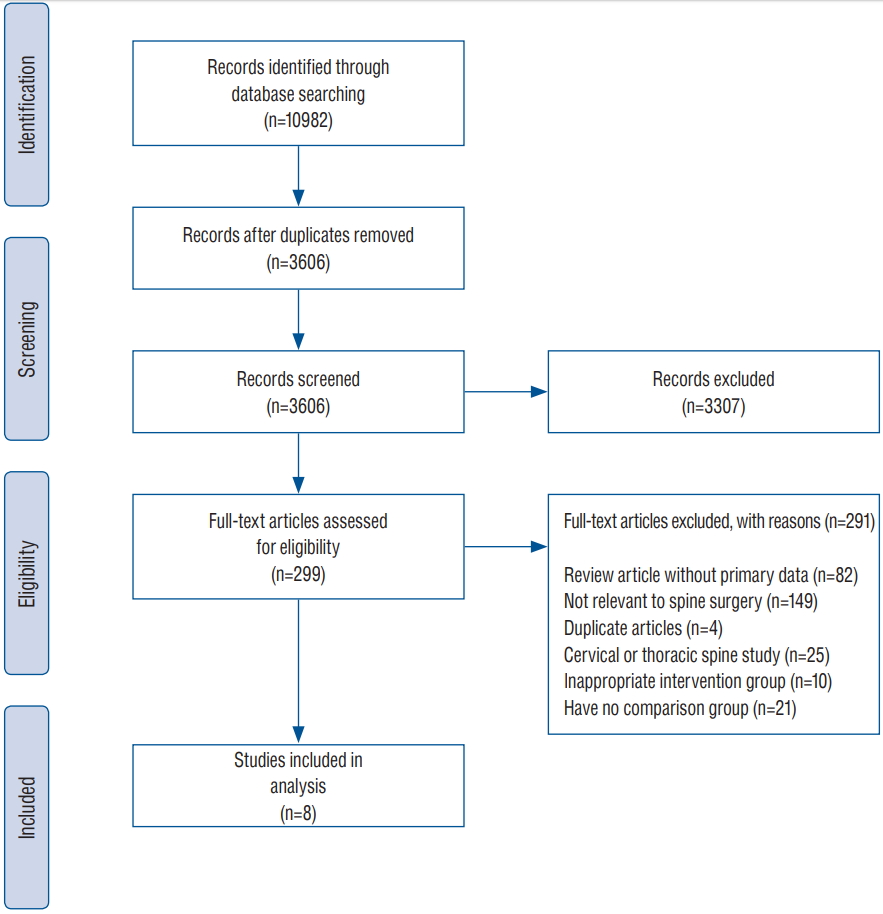
Fig. 1 shows the details of study identification, inclusion, and exclusion criteria. An electronic search yielded 2291 studies in PubMed (MEDLINE), 2572 in EMBASE, 144 in the Cochrane Library, 3379 in the Web of Science, and 2596 in Scopus. After removing duplicates, 3606 studies remained, of which 3592 were excluded after reviewing the abstracts and full-text articles. Therefore, eight studies were finally included in this meta-analysis [2,8,12,24,29,30,32,35].
Study characteristics and patient populations
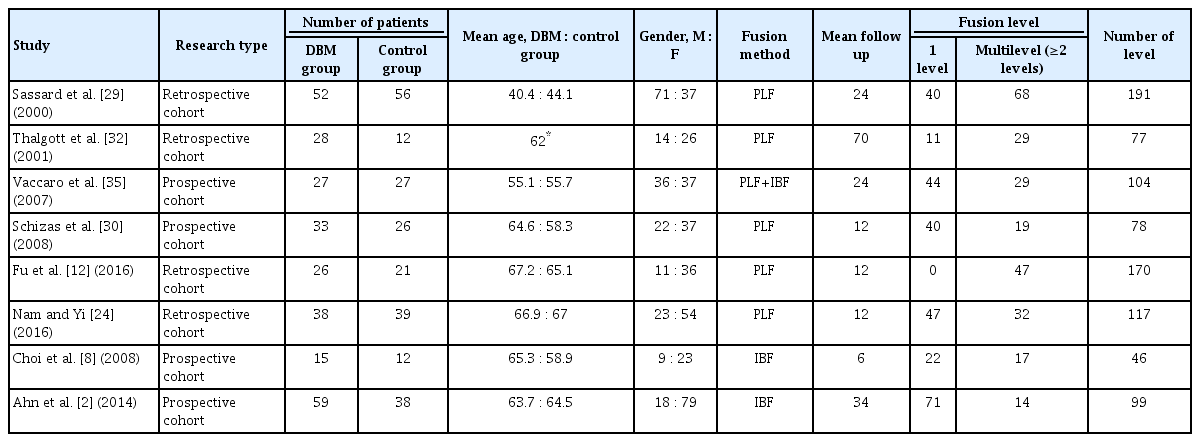
The meta-analysis included four prospective cohort studies, and four retrospective studies. There were six studies on posterolateral lumbar fusion surgery, two studies on lumbar interbody fusion, and one study on posterolateral lumbar fusion surgery and lumbar interbody fusion. These eight studies included 581 patients and 884 fusion levels. Among them, 377 patients underwent spinal fusion surgery using DBM (DBM group) and 204 patients underwent spinal fusion surgery with mainly autologous bone and without using DBM (control group). The details of the included studies are presented in Tables 1 and 2.
Quality of the included studies
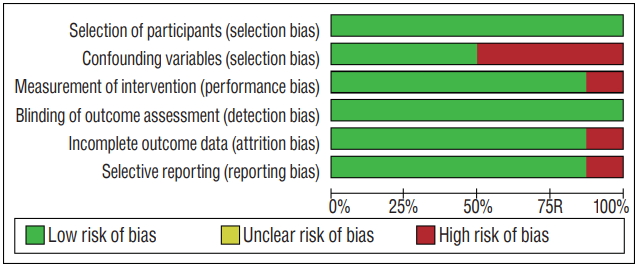
The results of quality assessment in eight studies included in this systematic review using RoBANS tool are summarized in Fig. 2. Overall, except for selection bias, these studies were rated as having low risk for other biases. Among the eight studies included in the assessment, 50% were evaluated as having low risk in the selection of participants and confounding variables. The low risk ratios of the performance bias, the attrition bias, and the reporting biases were 87.5%, respectively.
The rate of posterolateral lumbar fusion
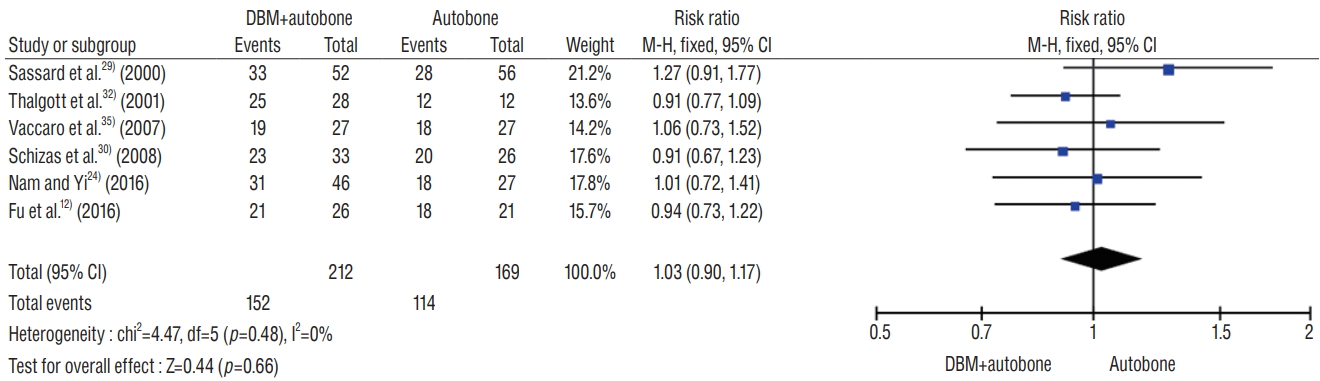
Of the eight studies included in the meta-analysis, six described the rate of posterolateral lumbar fusion. Six studies included 377 patients who underwent posterolateral lumbar fusion surgery and described the fusion incidence in the DBM group and the control group as 71.7% (152/212) and 67.5% (114/169), respectively. However, there was no significant difference in the fusion rate between two groups (odds ratio [OR], 1.03; 95% confidence interval [CI], 0.90–1.17; p=0.66). There was no heterogeneity among the selected studies evaluating the fusion rate (I2=0%, p=0.48) (Fig. 3).
The rate of lumbar interbody fusion
Of the eight studies included in the meta-analysis, three described the rate of lumbar interbody fusion as 70.1% (89/127) in the DBM group and 62.6% (48/77) in the control group, respectively. There was no significant difference in the fusion rate between two groups (OR, 1.13; 95% CI, 0.91–1.39; p=0.27). There was a heterogeneity among the selected studies evaluating the fusion rate (I2 =57%, p=0.10) (Fig. 4).
DISCUSSION
Autologous ICBG has been rated as the gold standard graft material in spinal fusion surgery [7,10]. However, it is known that there are several complications associated with the ICBG harvest [3,31]. Usage of recombinant BMP as the osteoinductive agent can achieve 94–99% fusion rate in spinal fusion surgery, but it has complications like radiculitis, ectopic bone formation, seroma formation, etc. [14,27]. To our knowledge, there are few reports about the complications due to usage of DBM as the bone graft in spinal fusion surgery.
Although DBM showed promising results in spinal fusion in animals, the results in humans were somewhat different [19,21,23,26]. The study by Cammisa et al. [6], which was a posterolateral lumbar fusion study in which DBM and autologous bone were inserted on one side and only autologous bone was inserted on the other side, reported that the fusion rate with DBM was similar to that with ICBG. Unlike this favorable result, Thalgott et al. [32] reported that the pseudarthrosis rate in the DBM group was higher than that in the DBM-less group. Overall, the efficacy of DBM in spinal fusion surgery is still controversial in view of the clinical results of DBM [9,22,25,34].
Therefore, we performed a meta-analysis to determine the effectiveness of DBM in spinal fusion surgery. First, in the observational studies of posterolateral lumbar fusion surgery, the overall fusion rate drops slightly in the DBM group compared to the control group. However, the fusion rate in the DBM group is not statistically different from that in the control group. Second, the results of lumbar interbody fusion surgery study were similar to those of the posterolateral lumbar fusion surgery study. These results may indicate that DBM is less effective as a graft enhancer than autologous bone. However, these studies suggest that DBM has an autologous bone -like graft extender capacity by showing a bone union rate similar to autologous bone.
This result was observed due to the characteristics of DBM. First, DBM is affected by the donor’s age, sex, and other unique characteristics which determine the efficacy of DBM [1]. Second, the manufacturing process of DBM differs from company to company. The intervariability and intravariability of BMP in each DBM product are detected by experimental procedures [4,5]. According to the study by Bae et al. [5], in each DBM product, concentration range of BMP-2 and BMP-7 are 20–120 ng/g and 54–226 ng/g, respectively. The quantity of BMP as the osteoinductive agent is too small in DBM product. Nevertheless, it is important to note that DBM has osteoinductive property. So, according to the result of this meta-analysis, DBM might have played role as a graft extender like autologous bone, rather than a graft enhancer.
Limitation of the study
This study has several limitations. Almost all the studies included in this meta-analysis are observational studies, suggesting inherent heterogeneity due to uncontrolled bias. Slight differences in different factors such as surgical skill, amount of graft material, sagittal balance, age, and density of bone, which could affect fusion rate may have increased the heterogeneity of the studies involved.
Some studies have included hydroxyapatite (HA) in the DBM group or autobone group. Particularly, since the components of HA do not contain BMP and their direct osteoinduction ability is substantially low, they are included in the study [17,18,28].
The fusion rate of posterolateral fusion is a bit higher than its interbody fusion. As a result of something different from other studies [20], the reason is mean follow-up duration. Follow-up duration is a little longer in posterolateral fusion studies than interbody fusion studies. Moreover, there is a variation in the number of surgical levels and it may be the confounding factor for this analysis. Additionally, the volume of DBM is different in each study, and two studies don’t describe the amount of DBM. Therefore, in terms of the volume of DBM, this meta-analysis study has heterogeneity and confounding factors.
Despite these limitations, this meta-analysis provides a snapshot of the best possible evidence currently available on the use of DBM in lumbar spinal fusion surgery.
Another limitation of this study is that there is no heterogeneity between studies of posterolateral fusion. This is only statistical point. Because the studies included in this study are observational studies, there is basically a heterogeneity between studies.
CONCLUSION
Based on the available evidence, the use of DBM with autograft in posterolateral lumbar spine fusion and lumbar interbody fusion showed a slightly higher fusion rate than that of autograft alone; however, there was no statistically different between two groups.
Notes
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : SH, SWC
Data curation : SH, DHK, DKA
Formal analysis : SH, BP
Methodology : SH, JWL
Project administration : SH, SWC
Visualization : SH
Writing - original draft : SH
Writing - review & editing : JYY, SWC
Acknowledgements
This work was supported by research fund of Chungnam National University.
Supplementary Materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2019.0185.
Electronic search strategy on each database.