Characteristics and Clinical Course of Fusiform Middle Cerebral Artery Aneurysms According to Location, Size, and Configuration
Article information
Abstract
Objective
To analyze the angiographic features and clinical course, including treatment outcomes and the natural course, of fusiform middle cerebral artery aneurysms (FMCAAs) according to their location, size, and configuration.
Methods
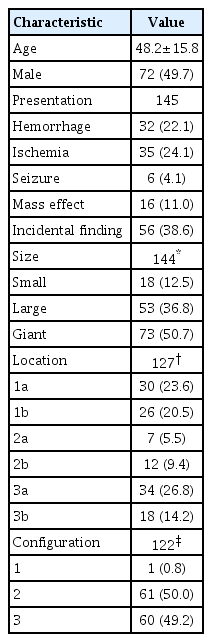
We reviewed the literature on adult cases of FMCAAs published from 1980 to 2018; from 25 papers, 112 FMCAA cases, for which the location, size, and configuration could be identified, were included in this study. Additionally, 33 FMCAA cases in our hospital were included, from which 16 were assigned to the observation group. Thus, a total of 145 adult FMCAA cases were included. We classified the FMCAAs according to their location (l-type 1, beginning from prebifurcation; l-type 2, beginning from bifurcation; l-type 3, beginning from postbifurcation), size (small, <10 mm; large, ≥10 mm; giant, ≥25 mm), and configuration (c-type 1, classic dissecting aneurysm; c-type 2, segmental ectasia; c-type 3, dolichoectatic dissecting aneurysm).
Results
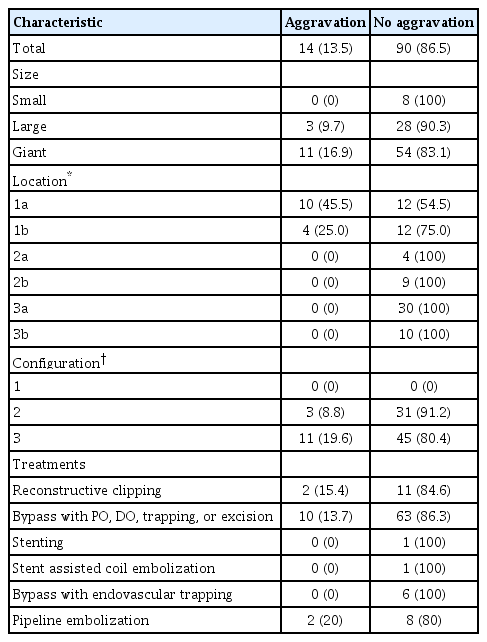
The c-type 3 was more commonly diagnosed with ischemic symptoms (31.8%) than hemorrhage (13.6%), while 40.9% were found accidentally. In contrast, c-type 2 was more commonly diagnosed with hemorrhagic symptoms (14.9%) than ischemic symptoms (10.6%), and 72.3% were accidentally discovered. According to location, ischemic symptoms and hemorrhage were the most frequent symptoms in l-type 1 (28.6%) and l-type 3 (34.6%), respectively. Most of l-type 2 FMCAAs were found incidentally (68.4%). Based on the size of FMCAAs, only 11.1% of small aneurysms were found to be hemorrhagic, while 18.9% and 26.0% of large and giant aneurysms were hemorrhagic, respectively. Although four aneurysms of the 16 FMCAAs in the observation group increased in size and one aneurysm decreased in size during the observation period, no rupture was seen in any case and there were no significant predictors of aneurysm enlargement. Of 104 FMCAAs treated, 14 cases (13.5%) were aggravated than before surgery and all the aggravated cases were l-type 1.
Conclusion
While ischemic symptoms occurred more frequently in l-type 1 and c-type 3, hemorrhagic rather than ischemic symptoms occurred more frequently in l-type 3 and c-type 2. In case of l-type 1 FMCAAs, more caution is required in determining the treatment due to the relatively high complication rate.
INTRODUCTION
Fusiform intracranial aneurysms (IAs) account for 3 to 13% of all IAs, with the vertebrobasilar system being their most common site of occurrence. Fusiform middle cerebral artery (MCA) aneurysms occur most frequently in the anterior circulation, followed by the internal carotid artery and the anterior cerebral artery [2,8,32,36]. The pathogenesis of fusiform IAs is known to be dissection, atherosclerosis, or connective tissue disease, and the clinical course of the disease has been reported to be related to its pathogenesis [30]. However, the pathogenesis and clinical course of fusiform MCA aneurysms (FMCAAs) are seldom known, although the occurrence of fusiform aneurysms in the vertebrobasilar system is relatively well known [10,18,22,27,29,39]. Especially, because of the low incidence of FMCAAs, there were few reports on their clinical course according to their location, size, and configuration, though patients with FMCAAs may present with various symptoms such as intracranial hemorrhage, cerebral ischemia, seizure, and mass effects depending on the location, size, and configuration [40].
We analyzed the angiographic features and clinical course, including treatment outcomes and the natural course, of FMCAAs according to their location, size, and configuration.
MATERIALS AND METHODS
Study population
This study was approved by the Institutional Review Boards of Seoul National University Bundang Hospital (B-1805/466-110). We reviewed the literature on adult FMCAA cases published from 1980 to 2018, and extracted only those documents from which the location, size, and shape could be identified. Finally, 112 FMCAA cases from 25 papers were included in this study (Table 1). In addition, we retrospectively analyzed adult patients diagnosed with FMCAAs who visited our hospital between 2003 and 2017. A total of 33 patients, 16 patients who had not undergone surgery and 17 patients who had undergone surgery, were included in this study. Accordingly, a total of 145 adult FMCAA cases were registered in this study to analyze their clinical and angiographic features according to their location, size, and configuration, and natural history was observed in 16 patients who had not undergone surgery. The baseline characteristics are presented in Table 2.
Location and size
The anatomical location of FMCAAs is an important determinant of the treatment modality, and it is generally classified as prebifurcation, bifurcation, and postbifurcation [17,34]. We divided the existing classification into more details based on the actual FMCAA location according to the following criteria : location type (l-type) 1a (located only at the prebifurcation), l-type 1b (beginning from the prebifurcation to postbifurcation), l-type 2a (located only at the bifurcation), l-type 2b (beginning from the bifurcation to postpostbifurcation), l-type 3a (beginning from postbifurcation and mainly located on M2), and l-type 3b (mainly located after M3 or M3) (Fig. 1).

Classification according to location of FMCAAs. l-type 1a, located only at prebifurcation; l-type 1b, beginning from prebifurcation to postbifurcation; l-type 2a, located only at bifurcation; l-type 2b, beginning from bifurcation to postpostbifurcation; l-type 3a, beginning from postbifurcation and mainly located on M2; l-type 3b, mainly located after M3 or M3. FMCAA : fusiform middle cerebral artery aneurysm.
Based on the size, the FMCAAs were classified as small (<10 mm in diameter), large (10–24 mm in diameter), and giant (diameter ≥25 mm) according to the conventional classification [6,40].
Configuration
Mizutani and Kojima [22] classified fusiform IAs according to angiographic and histopathological features as follows. Type 1 is a “classic dissecting aneurysm” showing a typical pseudolumen with packed fresh thrombus, and the portion of the pseudolumen corresponds to irregular stenosis on angiography. Angiography of such arterial dissections frequently shows irregular stenosis without aneurysmal dilatation (Fig. 2A). Type 2 is “segmental ectasia” appearing as luminal dilatation with distended or fragmented internal elastic lamina and thickened intima; thrombus formation does not occur in type 2 aneurysms. Angiography of this type shows fusiform aneurysm with smooth contour (Fig. 2B). Finally, type 3, “dolichoectatic dissecting aneurysm”, is characterized by tortuous fusiform appearance with irregular contrast due to luminal thrombus (Fig. 2C) [22]. We also classified the aneurysms based on Mizutani’s classification and named them as configuration type (c-type) 1, c-type 2, and c-type 3 to distinguish them from location types.
Clinical features
Clinical features were reviewed and analyzed for initial presentations, treatment outcomes, and natural history. The initial presentation was mainly an intracranial hemorrhage, cerebral ischemia, seizure, mass effect, or incidental finding. The mass effect was defined as the presence of a symptomatic lesion with brain shift, sulcal effacement, or cerebral edema on computed tomography or magnetic resonance imaging [21]. The treatment outcome was evaluated based on the modified Rankin Score (mRS) and classified as mild (0–1), moderate (2–3), and severe (4–5). We also analyzed whether the mRS deteriorated after surgery compared to before.
Natural history was observed in 16 patients who did not undergo surgery, and risk factor evaluation was performed in patients whose aneurysm size increased during follow-up. We performed statistical analysis using the SPSS statistical software (version 22; IBM Corp., Armonk, NY, USA). Categorical variables are presented as numbers and percentages. Continuous data are shown as mean±standard deviation. The Fisher exact test and Manne-Whitney U test were used to evaluate the risk factors for the increased size of FMCAAs. p-values less than 0.05 were considered to indicate statistical significance.
RESULTS
Characteristics according to location
In total, 44.1% of the FMCAAs (l-type 1a, 23.6%; l-type 1b, 20.5%) occurred at the prebifurcation (l-type 1) and 15.0% (2a, 5.5%; 2b, 9.4%) and 40.9% (3a, 27.0%; 3b, 13.9%) at the bifurcation (l-type 2) and postbifurcation (l-type 3), respectively (Fig. 3A). An analysis of the size of FMCAAs according to location revealed that the giant aneurysms accounted for 48.2%, 36.8%, and 46.2% of l-type 1, l-type 2, and l-type 3, respectively. Furthermore, large aneurysms accounted for 44.6%, 63.2%, and 30.8%, and small aneurysms, 7.1%, 0%, and 23.1% of l-type 1, l-type 2, and l-type 3, respectively (Fig. 3B). An analysis of the configuration of FMCAAs according to location revealed that 37.5% and 60.8% of l-type 1 were segmental ectasia type (c-type 2) and dolichoectatic dissecting type (c-type 3), respectively. Moreover, they accounted for 72.7% and 27.3%, respectively, in l-type 2 and 55.0% and 45.0%, respectively, in l-type 3 (Fig. 3C). An analysis of the initial symptoms depending on location revealed that the most common symptom in l-type 1 was ischemia (28.6%) and the second most common symptom was the mass effect (14.3%). The l-type 2 was more frequently found incidentally (68.4%) compared to other l-types and in case of l-type 3, hemorrhagic presentation (34.6%) was the most common, followed by ischemia (19.2%) (Fig. 3D).
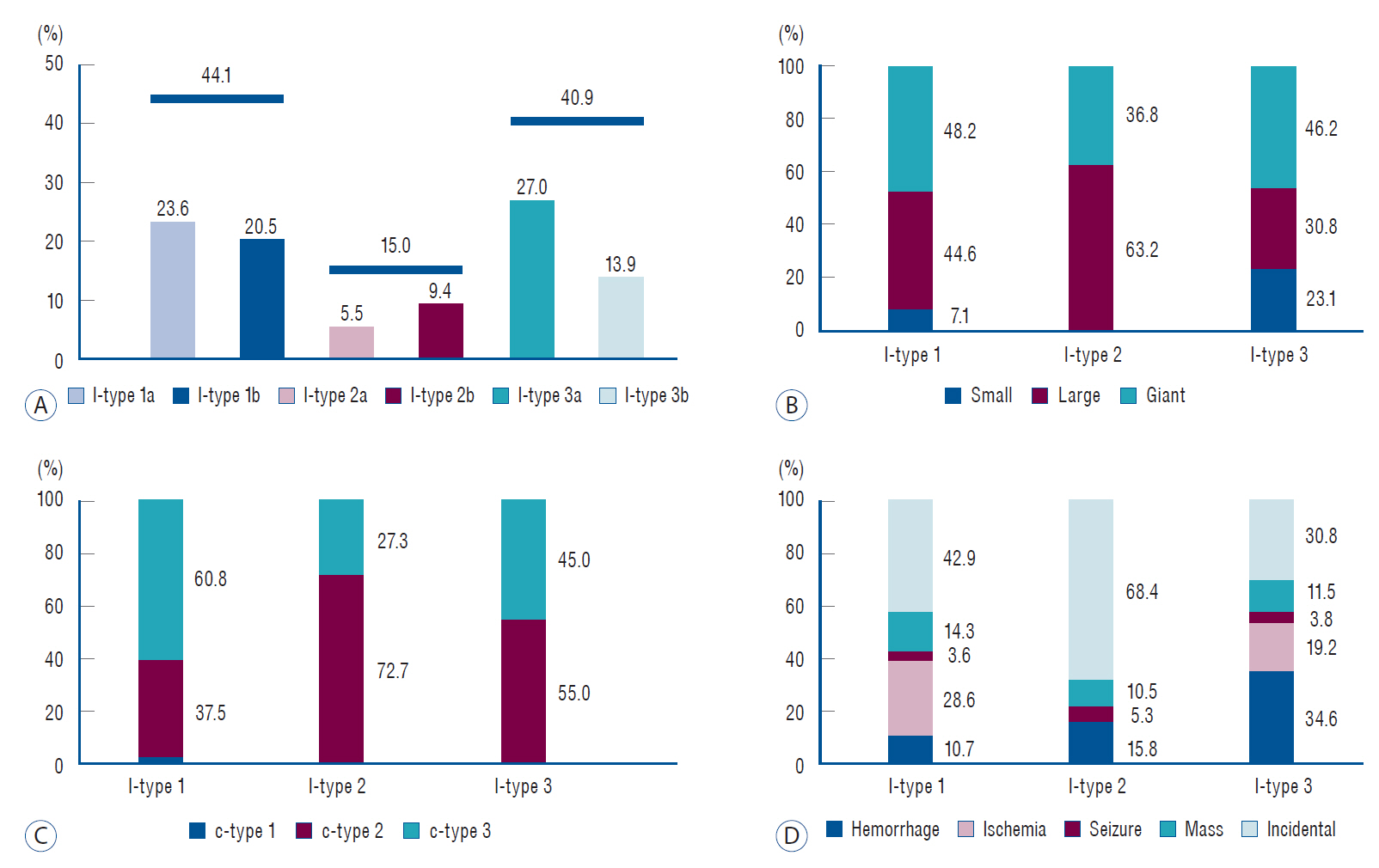
Characteristics according to location of FMCAAs. A : Frequency of occurrence of FMCAAs according to location. B : Distribution ratio of size of FMCAAs by location. C : Distribution ratio of configuration of FMCAAs by location. D : Clinical presentation according to location of FMCAAs. FMCAA : fusiform middle cerebral artery aneurysm.
Characteristics according to configuration
According to the analysis based on configuration, the ratio of c-type 2 and c-type 3 was similar at 49.7% and 48.9% (Fig. 4A). Regarding the size according to the configuration, the giant aneurysms accounted for largest proportion, 60.0%, in c-type 3, followed by large aneurysms (30.0%). On the other hand, the large aneurysms accounted for 68.9% of c-type 2, followed by small aneurysms, which accounted for 19.7% (Fig. 4B). Regarding the initial symptoms according to configuration, the rate of ischemia in c-type 3 was higher at 31.8%, while that of hemorrhage was lower at 13.6%. In contrast, the rate of hemorrhage in c-type 2 was higher at 14.9%, while that of ischemia was lower at 10.6% (Fig. 4C).

Characteristics according to location and size of FMCAAs. A : Frequency of occurrence of FMCAAs according to configuration. B : Distribution ratio of size of FMCAAs by configuration. C : Clinical presentation according to configuration of FMCAAs. D : Clinical presentation according to size of FMCAAs. FMCAA : fusiform middle cerebral artery aneurysm.
Characteristics according to size
While 61.1% of small aneurysms were discovered incidentally, only 28.8% of giant aneurysms were discovered incidentally. Moreover, only 11.1% of small aneurysms were found to be hemorrhagic, while 18.9% and 26.0% of large and giant aneurysms were hemorrhagic, respectively (Fig. 4D).
Natural course of FMCAAs
We observed a mean of 82.3±40.3 months for 16 aneurysms in 16 patients who had not undergone surgery; two aneurysms (12.5%) were giant aneurysms and 10 (62.5%) were large aneurysms. Of the 16 FMCAAs, four increased in size and one decreased in size during the observation period and the average period of increase in size was 106.1±48.9 months (11.8–129.5 months). Of the four FMCAAs that increased in size, two, which were c-type 3, showed arterial occlusion due to enlarged thrombus. One of them was l-type 2a and acute infarction was found in the temporal lobe because of one branch artery occlusion. The patient showed mild dysphasia, but eventually this improved (case 1, Fig. 5). The other one was l-type 3a that showed no symptoms despite the distal branch occlusion (case 2, Fig. 6). There has been no significant change in size since the increase was initially observed, as well as no rupture in any case. The classification of the four enlarged aneurysms by location and configuration is shown in Table 3. There were no significant predictors of aneurysm enlargement when analyzed by sex, age, underlying disease, size, location, and configuration.
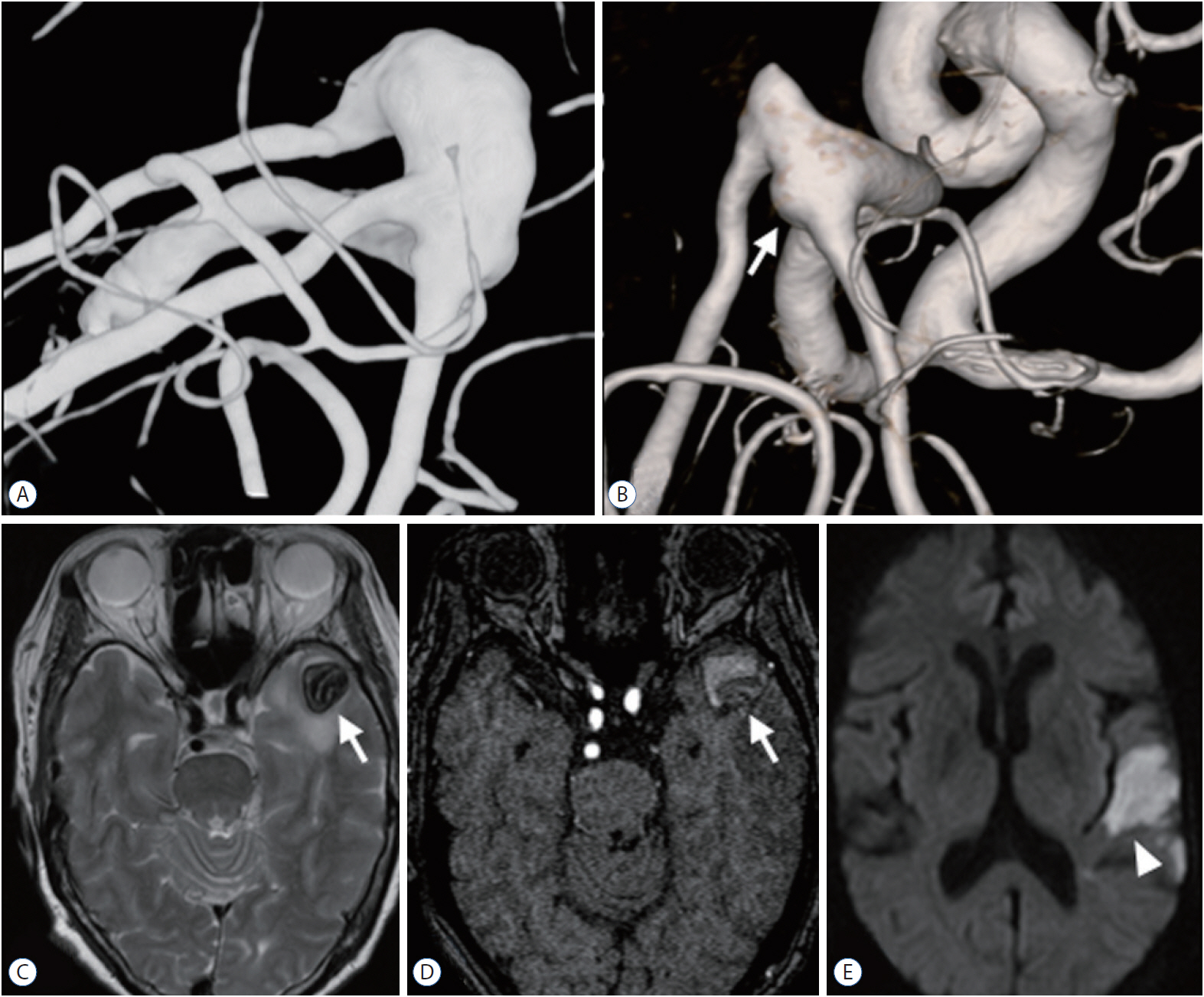
Case 1, 67-year-old woman. A and C : MRI and TFCA revealed that the FMCAA (white arrow) was classified as l-type 2a and c-type 3, and the maximum diameter was 19 mm. B : After 83 months, the occlusion of middle branch of postbifurcation MCA (white arrow) was found on TFCA. D and E : MRI showed the increased size of thrombosed aneurysm (white arrow) to 22 mm and detected the acute infarction (arrowhead) in the temporal lobe. MRI : magnetic resonance imaging, TFCA : transfemoral carotid angiography, FMCAA : fusiform middle cerebral artery aneurysm, MCA : middle cerebral artery.

Case 2, 50-year-old woman. A and B : A thrombosed fusiform aneurysm (white arrow) with maximum diameter of 18 mm was diagnosed and was classified as l-type 3a and c-type 3. C and D : One year later, the maximum diameter increased to 25 mm and distal artery occlusion was observed because of increased size of thrombus (white arrow).
Treatment outcomes of FMCAAs
Among the 121 FMCAAs treated, the difference between before and after treatment could be confirmed in 104 FMCAAs. Of the 104 FMCAAs, 86 (82.7%) were treated surgically, 12 (11.5%) were treated with endovascular procedures, and six FMCAAs (5.8%) with combined treatment. Overall, 14 (13.5%) cases were aggravated than before surgery. Among them, the cases of 9.7% of patients with large FMCAAs and 16.9% of patients with giant FMCAAs deteriorated compared with the preoperative status. Comparing by location, exacerbations were observed in 45.5% of l-type 1a and 25.0% of l-type 1b, but the patients with FMCAAs at other locations did not show deterioration. Additionally, comparing by shape, deterioration was observed in 8.8% of patients with c-type 2, and 19.6% patients with c-type 3 (Table 4).
Representative cases
Case 1
A thrombosed fusiform aneurysm located in the left MCA trifurcation was incidentally found in a 67-year-old woman. MRI and TFCA revealed that the FMCAA was classified as l-type 2a and c-type 3, and the maximum diameter was 19 mm. Surgery was recommended, but the patient refused and was treated conservatively. After 83 months, she was admitted to the emergency room with mild dysphasia, and acute infarction was diagnosed in the temporal lobe caused by branch artery occlusion. The maximum diameter of the FMCAA was increased to 22 mm. Conservative treatment was maintained and symptoms completely resolved. Thereafter, antiplatelets were administered and there was no further increase in size (Fig. 5).
Case 2
A thrombosed fusiform aneurysm in the left M2 artery was incidentally found in a 50-year-old woman. The FMCAA was classified as l-type 3a and c-type 3, and the maximum diameter was 18 mm. Surgery was recommended, but the patient refused and she was treated conservatively. One year later, the maximum diameter increased to 25 mm and distal artery occlusion was observed. Fortunately, there were no neurological symptoms, and there was no change afterwards (Fig. 6).
Case 3
A 72-year-old man with bladder cancer was found to have fusiform aneurysm in the left MCA bifurcation in tests conducted because of a headache. On MRI and TFCA, l-type 2a and c-type 2 were revealed, and maximum dimeter was 15 mm. Conservative treatment was decided, considering old age and underlying diseases. In the examination conducted 49 months later, the maximum diameter increased to 18 mm, but there was no rupture of aneurysm for 8 years until recently (Fig. 7).
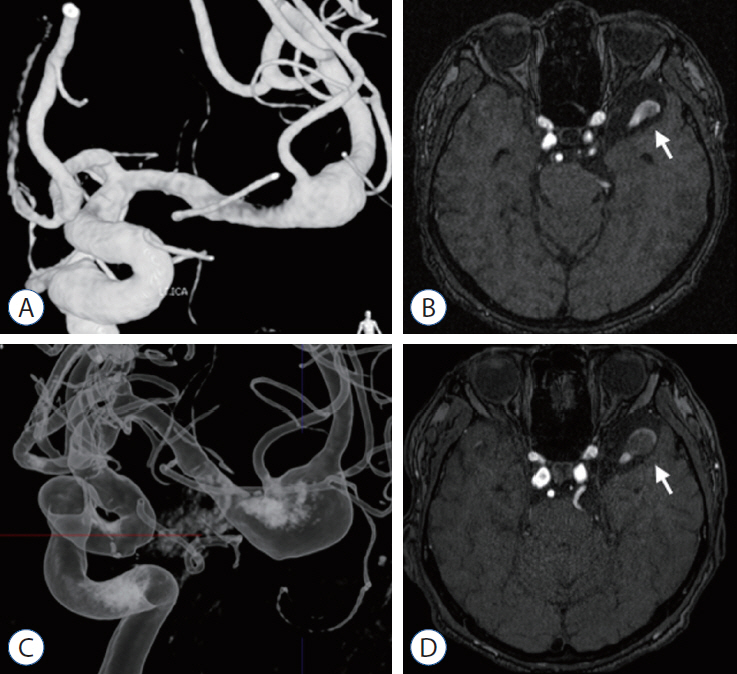
Case 3, 72-year-old man. A and B : FMCAA, classified as l-type 2a and c-type 2, was detected on TFCA and MRI (white arrow), and maximum dimeter was 15 mm. C and D : After 49 months, the maximum diameter increased to 18 mm (white arrow), but there was no rupture of aneurysm for 8 years until recently. FMCAA : fusiform middle cerebral artery aneurysm, TFCA : transfemoral carotid angiography, MRI : magnetic resonance imaging.
DISCUSSION
According to the present study, c-type 3 was more common with ischemic symptoms (31.8%) than hemorrhage (13.6%), while 40.9% of these FMCAAs were found accidentally. In contrast, c-type 2 was more common with hemorrhagic symptoms (14.9%) than ischemic symptom (10.6%), and 72.3% were discovered by accident. A previous meta-analysis for the natural history of vertebrobasilar dolichoectatic and fusiform aneurysms demonstrated that patients with fusiform aneurysms, which means c-type 2 in this study, had higher rupture rates than those with dolichoectatic aneurysms, which means c-type 3 in this study, and patients with dolichoectatic aneurysms had higher ischemic stroke rates than those with fusiform aneurysms, but this did not reach statistical significance [27]. Furthermore, Mizutani and Kojima [22] demonstrated that segmental ectasia type of fusiform aneurysm was usually asymptomatic and was incidentally diagnosed. In this study, c-type 3 was assumed to have more symptoms than c-type 2 because 60.0% of c-type 3 aneurysms were giant aneurysms in contrast to c-type 2 in which only 11.5% were giant aneurysm, as well as having intramural or intraluminal hematoma. In addition, the fact that 60.8% of l-type 1, in which most lenticulostriate arteries (LSA) are located [19], were c-type 3, can be a causative factor for ischemic symptoms.
Analyzing according to location, l-type 1 was found most frequently (44.1%) and the ratio of large aneurysm (44.6%) and giant aneurysm (48.2%) was higher in l-type 1 compared with that in l-type 3. The reason can be assumed as that the pathogenesis of FMCAAs was known as arterial dissection [6,30], and it has been reported that the shear stress that causes the dissection increases with the proximity of the region [35,37]. However, the reason why l-type 3 was reported more frequently than l-type 2 despite of lower proximity of the region is supposed that the MCA bifurcation is more susceptible site to saccular aneurysm than fusiform aneurysm. Analyzing the location-specific symptoms, 28.6% of l-type 1 cases showed ischemic symptoms, while 68.4% of l-type 2 were found by accident and 34.6% of l-type 3 were found as hemorrhage. The reason for this is that c-type 3, which can cause ischemia more frequently, was the most common (60.8%) in l-type 1, and c-type 2, in which hemorrhage is a more frequent symptom, was the most common (55.0%) in l-type 3. In the case of l-type 2, the proportion of giant aneurysms was relatively low (36.8%) and l-type 2 aneurysms were located in the border of the anterior and posterior compartments of the sylvian cistern where the cistern is wider [20]. This may explain why 68.2% of l-type 2 aneurysms were found incidentally without symptoms.
When analyzing the articles included in this study, l-type of FMCAA is the most important factor in determining the method of treatment. If LSA is involved in l-type 1, reconstructive clipping, proximal occlusion (PO) with bypass or distal occlusion with bypass is considered. If LSA is not involved, trapping with bypass or excision with bypass can be performed. However, if MCA bifurcation is involved, such as in l-type 1b, a combination bypass should be considered. In case of l-type 2a, reconstructive clipping can be considered, but l-type 2b was treated with trapping with combination bypass or PO with combination bypass. L-type 3 can be considered for trapping with bypass or excision with bypass. However, l-type 3a was treated with PO with bypass in cases located near the insular apex. In addition, endovascular treatment, such as stenting, stent-assisted coil embolization, or pipeline embolization, was performed for l-type 1 or l-type 3. Analyzing the results of the treatment, 13.5% (14 patients) deteriorated after the treatments, though among the 14 patients in whom there was aggravation after the treatments, nine (64.3%) were relatively healthy with a mRS score of 0 or 1 before the treatments. In addition, the cases that deteriorated after treatments were all l-type 1 and 36.8% of l-type 1 cases experienced aggravation after treatments.
In contrast, we observed the natural course of 16 FMCAA cases, and although the giant aneurysm was only included in two cases, there were no cases that ruptured during the observation period. In addition, although there were four cases in which the aneurysm increased in size, they did not increase after a certain period (11.8–129.5 months). Though blood pressure may have been thoroughly controlled after diagnosis of the aneurysm, this result shows that the natural course of the FMCAAs, especially that of c-type 2, is relatively benign. However, although our patients had improved symptoms, we need to be cautious in determining the observation of c-type 3 without treatments because of the presence of acute infarction caused by arterial occlusion.
Based on the above results, determining the course of treatment for patients with l-type 1 FMCAA with mRS scores of 0 or 1 requires caution because the natural course of FMCAAs is relatively benign compared to the poor results of surgery. However, in the case of conservative treatment without surgery, blood pressure control should be the most basic requirement, and l-type 1 or c-type 3 should be observed closely to prevent the aggravation of ischemic symptoms. In addition, for l-type 3 or c-type 2, it is also necessary to pay more attention to blood pressure control because of the relatively higher occurrence rate of hemorrhage when observation without surgery is undertaken. However, since FMCAA other than l-type 1 has a relatively good surgical outcome, surgery can be considered for l-type 2 and l-type 3, especially c-type 3, in institutes that can provide expert surgical experience.
Limitations
This study has some limitations because of its retrospective design. First, selection bias is one of the most important limitations because this study is based on previous published literature. It is likely that relatively severe cases were reported in case selection, and in therapeutic terms, treatment results were reported mainly in cases with good results. The fact that a natural course without treatment was observed only in 16 cases and that only two giant aneurysms and only four c-type 3 FMCAA were included were also limitations. Accordingly, it can be difficult to accurately understand the natural course of the FMCAAs that induce the symptoms clinically. However, because FMCAA is a rare disease and it is difficult to collect enough cases to study, the results of this study based on the previously reported cases will help to comprehensively identify FMCAA characteristics and predict their natural course.
CONCLUSION
The study analyzed the clinical characteristics of FMCAAs, which included outcome of treatments and the natural course, according to their location, configuration, and size. In conclusion, in cases of l-type 1 aneurysms, especially when accompanied by c-type 3, more caution is required in determining the treatment, and careful observation is needed to monitor the occurrence of ischemic symptoms when not treated. On the other hand, in case of l-type 3 and c-type 2, more attention should be paid to blood pressure control to prevent rupture of aneurysms. To obtain more accurate results, a prospective multicenter study is required in the future.
Notes
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : SUL, JSB
Data curation : DS
Formal analysis : TK
Funding acquisition : JSB
Methodology : SPB, HSB
Project administration : CWO, OK
Visualization : YDW, YDK, YL
Writing - original draft : DS, SUL
Writing - review & editing : SUL
Acknowledgements
This study was supported by the Research Funds of the Seoul National University Bundang Hospital.