Reduction of Inflammation and Enhancement of Motility after Pancreatic Islet Derived Stem Cell Transplantation Following Spinal Cord Injury
Article information
Abstract
Objective
Spinal cord injury (SCI) is a very serious health problem, usually caused by a trauma and accompanied by elevated levels of inflammation indicators. Stem cell-based therapy is promising some valuable strategies for its functional recovery. Nestin-positive progenitor and/or stem cells (SC) isolated from pancreatic islets (PI) show mesenchymal stem cell (MSC) characteristics. For this reason, we aimed to analyze the effects of rat pancreatic islet derived stem cell (rPI-SC) delivery on functional recovery, as well as the levels of inflammation factors following SCI.
Methods
rPI-SCs were isolated, cultured and their MSC characteristics were determined through flow cytometry and immunofluorescence analysis. The experimental rat population was divided into three groups : 1) laminectomy & trauma, 2) laminectomy & trauma & phosphate-buffered saline (PBS), and 3) laminectomy+trauma+SCs. Green fluorescent protein (GFP) labelled rPI-SCs were transplanted into the injured rat spinal cord. Their motilities were evaluated with Basso, Beattie and Bresnahan (BBB) Score. After 4-weeks, spinal cord sections were analyzed for GFP labeled SCs and stained for vimentin, S100β, brain derived neurotrophic factor (BDNF), 2’,3’-cyclic-nucleotide 3'-phosphodiesterase (CNPase), vascular endothelial growth factor (VEGF) and proinflammatory (interleukin [IL]-6, transforming growth factor [TGF]-β, macrophage inflammatory protein [MIP]-2, myeloperoxidase [MPO]) and anti-inflammatory (IL-1 receptor antagonis) factors.
Results
rPI-SCs were revealed to display MSC characteristics and express neural and glial cell markers including BDNF, glial fibrillary acidic protein (GFAP), fibronectin, microtubule associated protein-2a,b (MAP2a,b), β3-tubulin and nestin as well as antiinflammatory prostaglandin E2 receptor, EP3. The BBB scores showed significant motor recovery in group 3. GFP-labelled cells were localized on the injury site. In addition, decreased proinflammatory factor levels and increased intensity of anti-inflammatory factors were determined.
Conclusion
Transplantation of PI-SCs might be an effective strategy to improve functional recovery following spinal cord trauma.
INTRODUCTION
Spinal cord injury (SCI) is a detrimental event mainly caused by a trauma [7], that can lead to physical disability including failure in motor, sensory or autonomic function [35]. SCI affects patients physically and psychologically as well as their financial condition [15]. SCI respectively causes edema, decreased blood flow, vasospasm, free radical production, inf lammation, excito toxicity, lipid peroxidation and finally ischemia provoke cell apoptosis [46]. Due to the non-responsive environment of the injured spinal cord, axon regeneration does not occur [58]. Besides, the loss of function after SCI might be caused by both the primary mechanical insult and multifaceted secondary degenerative response [50].
Some experimental studies in the last decades, proved that the injured spinal cord could be restored [50]. Nowadays, stem cell based therapy is promising some valuable strategies for functional recovery of the injured spinal cord [62]. In this context, mesenchymal stem cells (MSCs) were used in addition to neural progenitor stem cells for functional recovery [40].
The mechanism of stem cell therapy in neurodegenerative diseases include support to neuronal growth, replacement of neuronal cells, preservation of glial cells, in creasing trophic molecules, remyelination of axons, regeneration of damaged synaptic connections [33,51]. Stem cells also have the potential for angiogenesis, bridging of cavities, reducing inflammation and stimulation of endogenous precursor cells for neuro nal plasticity [33] besides their anti-apoptotic effect [51]. In addition to their capability of differentiation and renewal, stem cells secrete substances that promote neuroprotection, such as cytokines, growth factors and trophic factors [54].
MSCs have become one of the crucial cell sources for the treatment of neurodegenerative conditions [24]. The source for MSC for therapeutic purposes can be the bone marrow [23], cord blood [30], adipose tissues [14], and dermis [39].
First described by Zulewski et al. [63], nestin-positive progenitor and/or stem cells (SC) isolated from human and murine pancreas have been shown to include phenotypic markers identical to MSCs [11,63]. These cells have the capability of proliferating and differentiating into insulin-producing cells, mesodermal and ecto-mesodermal germ layer derived somatic cells such as adipocytes and osteocytes in vitro [63]. Additionally, nestin positive MSCs are considered to be a reliable source for central nervous system (CNS) repair [31].
Besides being a derivation of embryonic endoderm, pancreatic islets share similar phenotypic traits with neurons [13]. In addition to the presence of insulin gene transcription in the vertebrate brain [12], recent studies suggest that pancreatic beta cells share common alternative splicing regulators and programs with neurons [25], proving that similarities continue at post-transcriptional level as well. Moreover, mouse pancreatic epithelial cells can give rise to neuron-like cells [44].
Rat pancreatic islet derived stem cell (rPI-SCs) have been reported to represent the characteristics of MSCs [47]. In our previous studies, we have also demonstrated the expression of neurogenic (eno2, microtubule associated protein-2a,b, c-fos, nestin, glial fibrillary acidic protein [GFAP], and β3-tubulin) and osteogenic (osteonectin, osteocalcin, osteopontin, runx2, bone morphogenetic protein [BMP]-2, BMP-4, and type-I collagen) markers in rPI-SCs [26].
In this study, we aimed to investigate the effects of rPI-SCs transplantation on functional recovery and neural regeneration processes following SCI, as well as reduction of proinflammatory factors within the injured spinal cord.
MATERIALS AND METHODS
Animals
The SCI study included about 2–3 months old 15 female, nonpregnant and five male Wistar albino rats with a weight of 200–300 g. In the first step of the study, five rats (male) were sacrificed in order to obtain rPI-SCs. The remaining rats were divided into three groups (five rats per group) : laminectomy+trauma (group 1), laminectomy+trauma+phosphate-buffered saline (PBS) (group 2); laminectomy+trauma+SCs (group 3). Rats were sacrificed 4 weeks after transplantation. The Ethics Committee of Kocaeli University approved the experimental design and all procedures with a IACUC protocol number of KOU/HAYDEK 1/2/2013.
Culture of rPI-SCs
The pancreatic islets were isolated as described previously [26] and cultured in RPMI 1640 (Invitrogen/GIBCO, Grand Island, NY, USA) with glucose 2 g/L supplemented with 10% fetal bovine serum (FBS; Invitrogen/GIBCO), 100 IU/mL penicilin-100 μg/mL streptomycin (Invitrogen/GIBCO) and glutamine (2 mmol/L; Invitrogen/GIBCO) at 37℃ in a humidified air atmosphere containing 5% CO2. Some islets immediately adhered to the surfaces of the flasks. Within several days, a monolayer of cells was observed growing out and away from the islets and after 13 to 15 days of culturing, cells in the monolayer reached to 70% confluency and named as passage zero (P0) cells. For passaging, the cells were washed with Ca2+-Mg2+ free phosphate-buffered saline (PBS) (Invitrogen/GIBCO) and detached by incubating with 0.25% trypsin-ethylenediaminetetraacetic acid solution (Invitrogen/GIBCO) for 5–10 minutes at 37℃. After addition of growth medium to inactivate trypsin, the cells were then centrifugated at 200 g for 10 minutes, resuspended in 1 mL complete medium, counted in duplicate using Thoma chamber and then plated in 75 cm2 flasks (BD Biosciences, San Diego, CA, USA) at densities of 1×106 cells/flask. The growth medium was replaced every 3 days over a 10–14 day period.
Flow cytometry
To confirm that rPI-SCs maintain their phenotypic characteristics after growth in culture, undifferentiated SCs were subjected to flow cytometry analysis. The surface markers of rPI-SCs at passages 3 (P3) were assayed with antibodies against the following rat antigens : CD29 (integrin β1 chain), CD45 (leukocyte common antigen), CD54 (intercellular adhesion molecule-1), CD90 (Thy-1/Thy-1.1), CD106 (vascular cell adhesion protein-1), major histocompatibility complex (MHC) classes I and II and their three isotype controls (IgG2a, κ).
All of the antibodies were supplied by Becton Dickinson (BD Biosciences). Flow cytometry was performed using a FACSCalibur (BD Biosciences). The data were analyzed with Cell Quest software (BD Biosciences).
Labeling with green fluorescent protein (GFP) of rPI-SCs
Green fluorescent protein (GFP) (Clontech, Palo Alto, CA, USA) was transfected by electroporation (Neon Transfection System; Invitrogen, Carlsbad, CA, USA) with respect to the instructions provided by the manufacturer. The transformed cells were cultured in 1 mL minimum essential media-medium with 15% FBS. After 48 hours of incubation, the cells were selected with respect to the resistance against G418 (200 μg/mL).
Surgical procedure and cell transplantation
For skin preparation of T10–T11 spinal cord surgery, lumbar laminectomy of tracer injection, dermal surface of the related regions was cleared by hair razor, and the skin was washed by antibacterial soap followed with betadine and 70% ethanol application [8]. After an overnight fast with unrestricted access to water, all 15 rats were anesthetized with intramuscular ketamine (50 mg/kg) and xylazine (5 mg/kg) prior to surgery. Under dissection stereomicroscope; 3 mm long laminectomy, encompassing the caudal end of T10 vertebra and the rostral end of T11 vertebra, was performed. For SCI groups (total, 15), after the laminectomy, the animals were moved to stabilization platform. The spine was immobilized stabilizing clamps and a severe T10–T11 contusive injury was introduced by dropping the impounder rod (1 g) from a height of 50 mm. The muscle and fascia layers were sutured and the skin was stapled subsequently.
GFP labeled rPI-SCs (3×105 cells/5 μL) were transplanted into the injured spinal cord via Hamilton syringe (Hamilton Company, Reno, NV, USA) connected to a syringe pump (KD Scientific Inc., Holliston, MA, USA) for 5 minutes, respectively. PBS group received 5 μL of PBS at the injured spinal cord with the same technique. The needle was removed 10 minutes after intraspinal transplantation, and muscle & skin layers were closed in layers. The bladders of SCI rats were evacuated twice daily during the entire study.
Beattie and Bresnahan (BBB) scoring-functional tests
Functional tests were performed using the BBB locomotor rating scale at pre-surgery, at days 1, 7, 14, 21, and 28 postinjury (p.i.). Two independent, blinded examiners observed each animal for 4 minutes. Hindlimb movements were recorded by video camera and locomotor functions were assessed [5]. The BBB scores were presented as mean±standard error.
Tissue harvesting and immunoflourescence examination
At the end of 4 weeks, rats were anesthetized with ketamine (75 mg/kg, i.p.) and xylazine (20 mg/kg, i.p.) and transcardially perfused with saline (150 mL/per animal) and followed with 4% neutral buffered paraformaldehyde in 0.1 mol/L PBS, pH 7.4. One cm spinal cord segment encompassing the injury site was removed, the tissues were post-fixed in 4% paraformaldehyde approximately 24 hours and then tissues were dehydrated through a graded series of ethanol, cleared with xylene and finally embedded in paraffin wax.
To perform cell tracing after injection of the GFP labeled rPI-SCs, an immunof luorescence double staining protocol was performed on sections. Slides were deparaffinized with two changes of xylene for 5 minutes each and rehydrated in a series of graded alcohol solutions. Sections were antigen retrieved using a steamer-citrate buffer antigen retrieval method. Endogenous peroxidases were inhibited by incubation with fresh 3% H2O2 in PBS buffer. Nonspecific staining was blocked with the mixture of two different serums at 1.5% in PBS for 30 minutes at room temperature. The sections were incubated in a mixture of two primary antibodies (Table 1) in a pairwise fashion with the mouse monoclonal anti-GFP antibody (sc-9996) for 1 hour at room temperature and appropriate secondary antibodies 30 minutes at room temperature. The mounted cells with mounting medium containing DAPI (Santa Cruz Biotechnology, Santa Cruz, CA, USA), were examined under fluorescence microscope. Immunoflourescence stainings on the P3 cells were performed as previously described [26]. The evaluation of the immunoflourescence stainings were accomplished by ImageJ program (National Institutes of Health, Bethesda, MD, USA) as described previously [49]. Briefly, corrected total cell fluorescence was determined through the integrated density, area and the mean gray value of the cells.
All experiments were repeated a minimum of three times. All data presented as mean±standard error. All statistical analyses were performed using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA). The data were analyzed using one-way analysis of variance. Differences between groups were regarded as statistically significant when p<0.05.
RESULTS
Isolation, culture and phenotype identification of SCs from rPIs
Examination of cultured islets under an inverted microscope showed that they had regular cellular structures (Fig. 1A). rPI-SCs spread on culture flask from islets and attached to the culture f lasks sparsely, and the majority of cells displayed a fibroblast-like, spindle-shaped morphology during the early days 20 of explant culture incubation. rPI-SCs reached a monolayer conf luence in the primary culture on 12–15 days 1 of being plated in their first passages. Most of the rPI-SCs exhibited large, flattened or fibroblast-like morphology in the later passages (Fig. 1B and C). We confirmed that rPI-SCs maintained their phenotypic characteristics after growth in culture and undifferentiated SCs were subjected to flow cytometry. Flow cytometry analysis revealed that, specific markers for MSCs were expressed by rPI-SCs such as CD29 (99.57%), CD90 (91.79%), CD54 (98.29%), MHC class I (73.77%). On the other hand, rPI-SCs did not express some of hematopoietic stem cell markers including CD45 (0.12%) and MHC class II (0.01%) and also CD106 (6.90%) (Fig. 1D).

Morphological characteristics of the rat pancreatic islets. Free floating rat pancreatic islets (A). Fibroblast-like cells are observed growing out and away from a pancreatic islets (B). rPI-SC morphologies (unstained) for late passage (C, P3–4th day). Flow cytometry analysis for P3 cells (C and D). Scale bars, 100 μm. FITC : fluorescein isothiocyanate, rPI-SC : rat pancreatic islet derived stem cell.
Immunoflourescence analysis revealed that, in addition to MSC markers such as vimentin (Fig. 2D), isolated rPI-SCs also expressed neural and glial cell markers including brain derived neurotrophic factor (BDNF) (Fig. 2A), GFAP (Fig. 2B), fibronectin (Fig. 2C), microtubule associated protein-2a,b (MAP2a,b) (Fig. 2E), β3-tubulin (Fig. 2F), and nestin (Fig. 2G). These cells were also expressing interleukin-1 receptor antagonis (IL-1ra) (Fig. 2H) and EP3 (Fig. 2I), inhibitors of pro-inflammatory cytokines (Fig. 2).
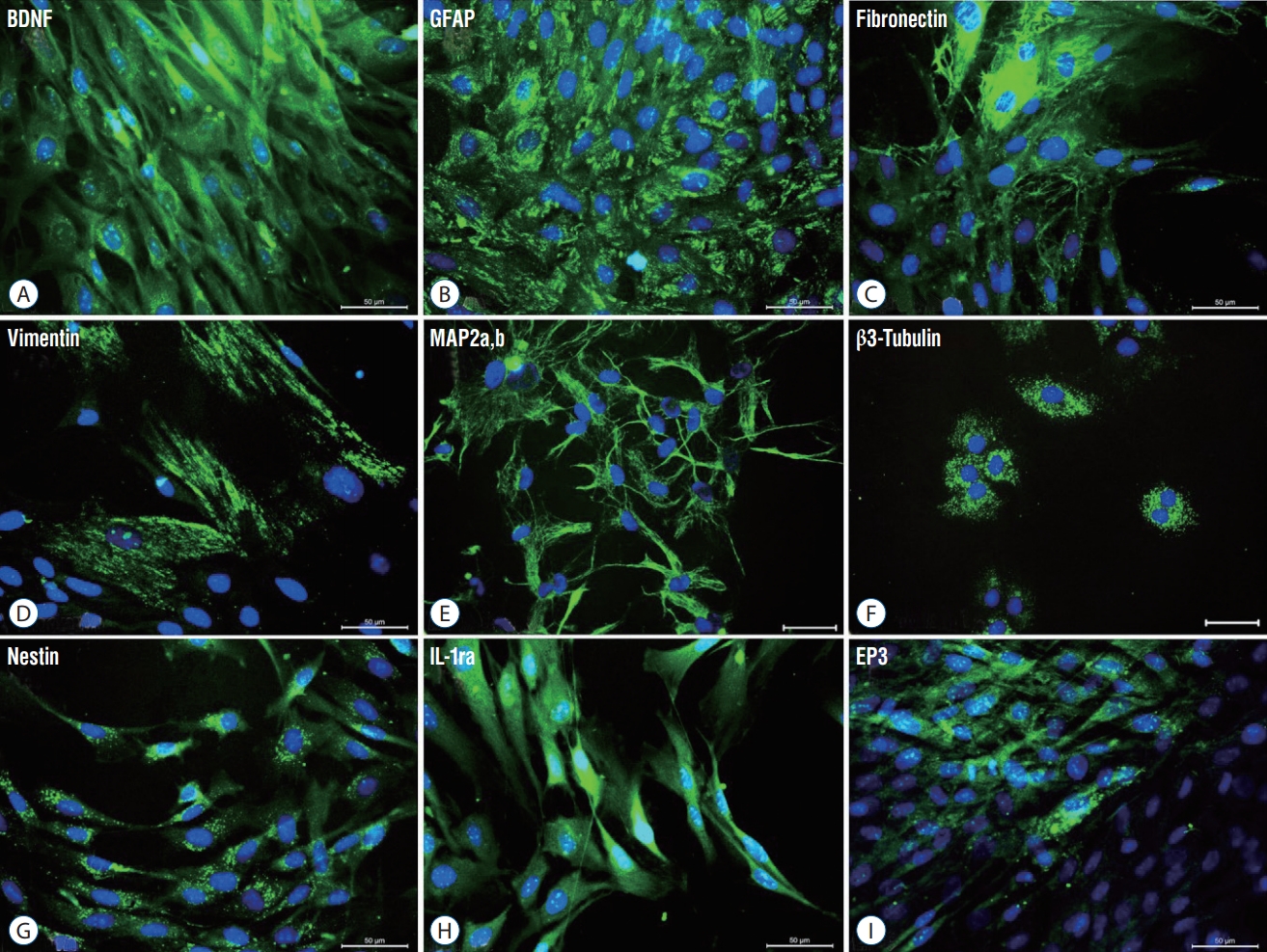
Representative panels of immunofluorescence stainings for phenotype identification of rPI-SC. The expression of cell markers of neurogenic (A-C and E-G) and mesenchymal markers (D) and inhibitors of pro-inflammatory cytokines (H, I). All markers were detected with FITC (green) labelled secondary antibodies. Nuclei were labeled with DAPI (blue) (Scale bars, 50 µm). BDNF : brain derived neurotrophic factor, GFAP : glial fibrillary acidic protein, MAP2a,b : microtubule associated protein-2a,b, IL-1ra : interleukin-1 receptor antagonis, FITC : fluorescein isothiocyanate, rPI-SC : rat pancreatic islet derived stem cell.
Survival and migration of rPI-SCs
Four weeks after rPI-SC transplantation, the immunofluorescence microscopic analysis of transversal sections of rat spinal cords from experimental groups were performed with double staining of GFP together with vimentin, β3-tubulin, 2’,3’-cyclic-nucleotide 3’-phosphodiesterase (CNPase), S100β, nestin, vascular endothelial growth factor (VEGF) or BDNF. In all sections of laminectomy+trauma groups, they showed negative staining for GFP (Figs. 3-7), as well as laminectomy & trauma & PBS group (data not shown). However, GFP+ cells were observed near the damage site of laminectomy+trauma+ SC group at the end of 4 weeks (Figs. 3-7). GFP+ SCs migrated into cavitated area from the injection sites and the majority of these still survived and expressed some stem cell and neural cell markers such as vimentin (Fig. 3), CNPase (Fig. 4), S-100β (Fig. 5), BDNF (Fig. 6), and also VEGF (Fig. 7A). Immunostaining intensity level of these factors were higher in laminectomy+trauma +SC group compared to laminectomy+trauma group (Fig. 7B) and laminectomy+trauma+PBS group (data not shown).

Immunofluorescence stainings in paraffin sections of rat spinal cord tissues. Four weeks after rPI-SC transplantation, GFP+ cells were migrated to the damaged site and survived in laminectomy+trauma+SC (group 3) animals’ sections. GFP+/vimentin+rPI-SCs were located in damaged area. Green : GFP, red : vimentin. Nuclei were labeled with DAPI (blue) (Scale bars, 50 µm). DAPI : 4',6-diamidino-2-phenylindole, dihydrochloride, GFP : green fluorescent protein, SC : stem cell, rPI-SC : rat pancreatic islet derived stem cell.
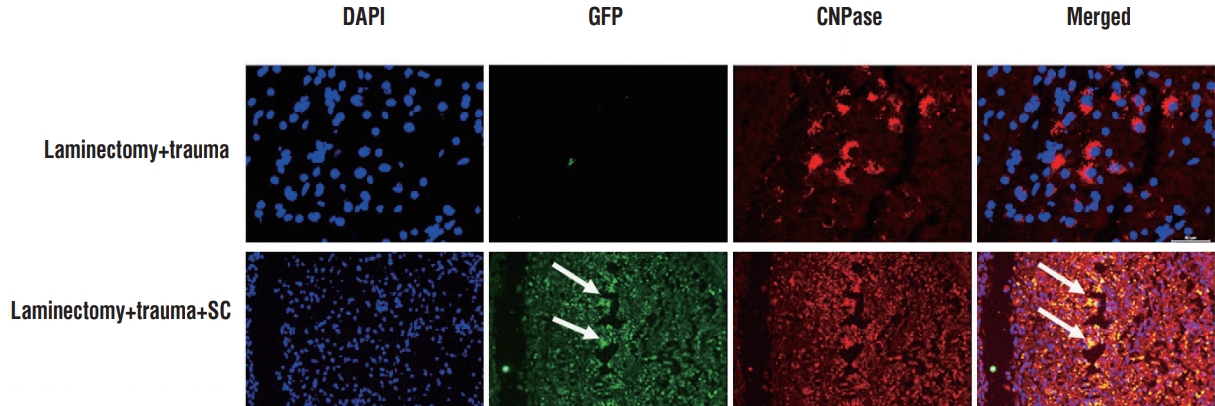
Immunofluorescence stainings in paraffin sections of rat spinal cord tissues. Four weeks after rPI-SC transplantation, GFP+ cells were migrated to the damaged site and survived in laminectomy+trauma+SC (group 3) animals’ sections. GFP+/CNPase+rPI-SCs were located in damaged area. Green : GFP, red : CNPase. Nuclei were labeled with DAPI (blue). Arrows indicate GFP+ cells. Scale bars is 50 µm. DAPI : 4',6-diamidino-2-phenylindole, dihydrochloride, GFP : green fluorescent protein, CNPase : 2’,3’-cyclic-nucleotide 3'-phosphodiesterase, SC : stem cell, rPI-SC : rat pancreatic islet derived stem cell.

Immunofluorescence stainings in paraffin sections of rat spinal cord tissues. Four weeks after rPI-SC transplantation, GFP+ cells were migrated to the damaged site and survived in laminectomy+trauma+SC (group 3) animals’ sections. GFP+/S100+rPI-SCs were located in damaged area. Green : GFP, red : S100β. Nuclei were labeled with DAPI (blue). Arrows indicate GFP+ cells. Scale bars is 50 µm. DAPI : 4',6-diamidino-2-phenylindole, dihydrochloride, GFP : green fluorescent protein, SC : stem cell, rPI-SC : rat pancreatic islet derived stem cell.

Immunofluorescence stainings in paraffin sections of rat spinal cord tissues. Four weeks after rPI-SC transplantation, GFP+ cells were migrated to the damaged site and survived in laminectomy+trauma+SC (group 3) animals’ sections. GFP+/BDNF+rPI-SCs were located in damaged area. Green: GFP, red : BDNF. Nuclei were labeled with DAPI (blue). Arrows indicate GFP+ cells. Scale bars is 50 µm. DAPI : 4',6-diamidino-2-phenylindole, dihydrochloride, GFP : green fluorescent protein, BDNF : brain derived neurotrophic factor, SC : stem cell, CNPase : 2’,3’-cyclic-nucleotide 3'-phosphodiesterase, rPI-SC : rat pancreatic islet derived stem cell.
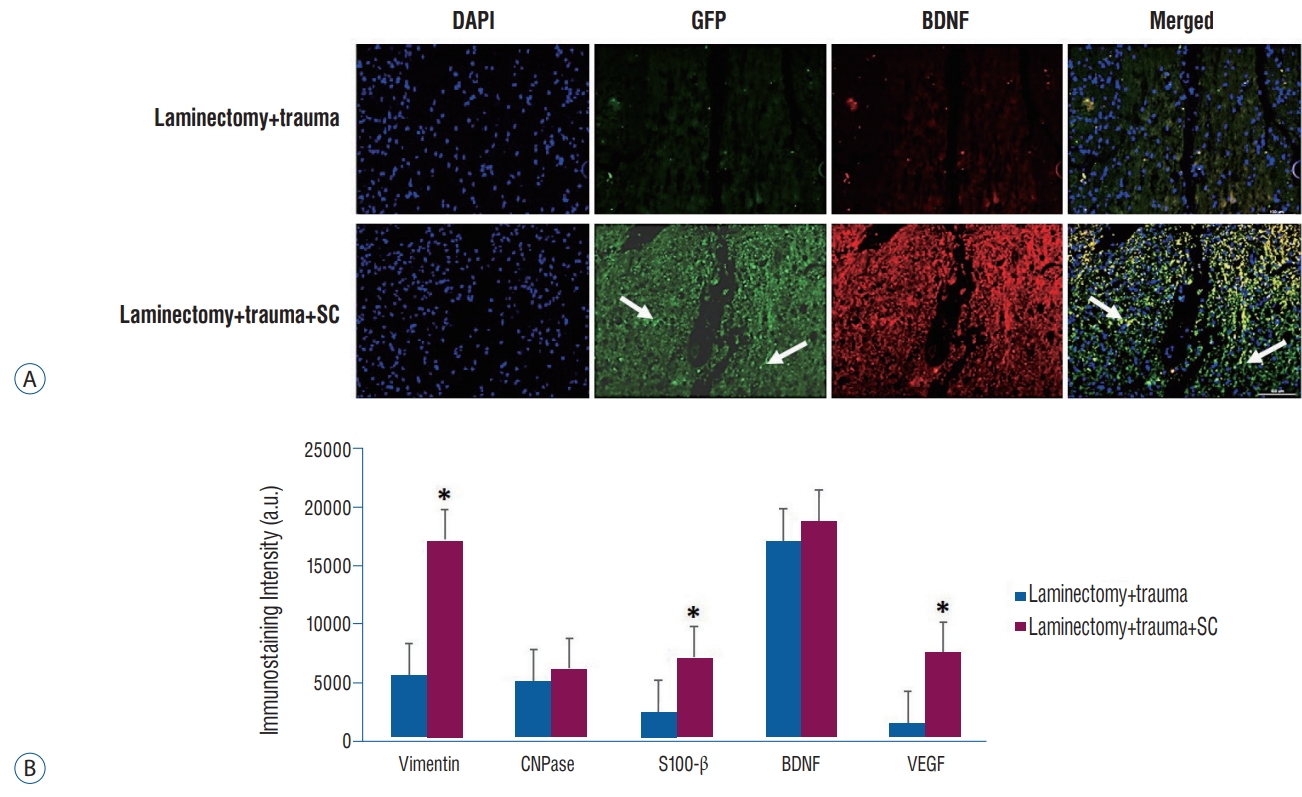
A : Immunofluorescence stainings in paraffin sections of rat spinal cord tissues. Four weeks after rPI-SC transplantation, GFP+ cells were migrated to the damaged site and survived in laminectomy+trauma+SC (group 3) animals’ sections. GFP+/VEGF+rPI-SCs were located in damaged area. Green : GFP, red : VEGF. Nuclei were labeled with DAPI (blue). Arrows indicate GFP+ cells. Scale bars is 50 µm. B : Graphs of immunostaining intensities. The values were presented as mean±standard error. *p<0.05. DAPI : 4',6-diamidino-2-phenylindole, dihydrochloride, GFP : green fluorescent protein, VEGF : vascular endothelial growth factor, SC : stem cell, BDNF : brain derived neurotrophic factor, a.u. : arbitrary unit, rPI-SC : rat pancreatic islet derived stem cell.
Distribution of inflammatory and anti-inflammatory factors in the injured spinal cord
Immunoflouresence analysis was applied to rat spinal cord sections of experimental groups. It was revealed that IL-1ra, a suppressor of proinflammatory cytokine IL-1 expression was higher in the spinal cords of rPI-SC injected group compared to the spinal cords of the rats that did not receive rPI-SCs. Conversely, IL-6, transforming growth factor (TGF)-β1, macrophage inflammatory protein (MIP)-2 and myeloperoxidase (MPO), indicators of inflammation, were expressed in a higher level in spinal cords of rPI-SC cell injected rats in compared to the ones without rPI-SC injection (Fig. 8).
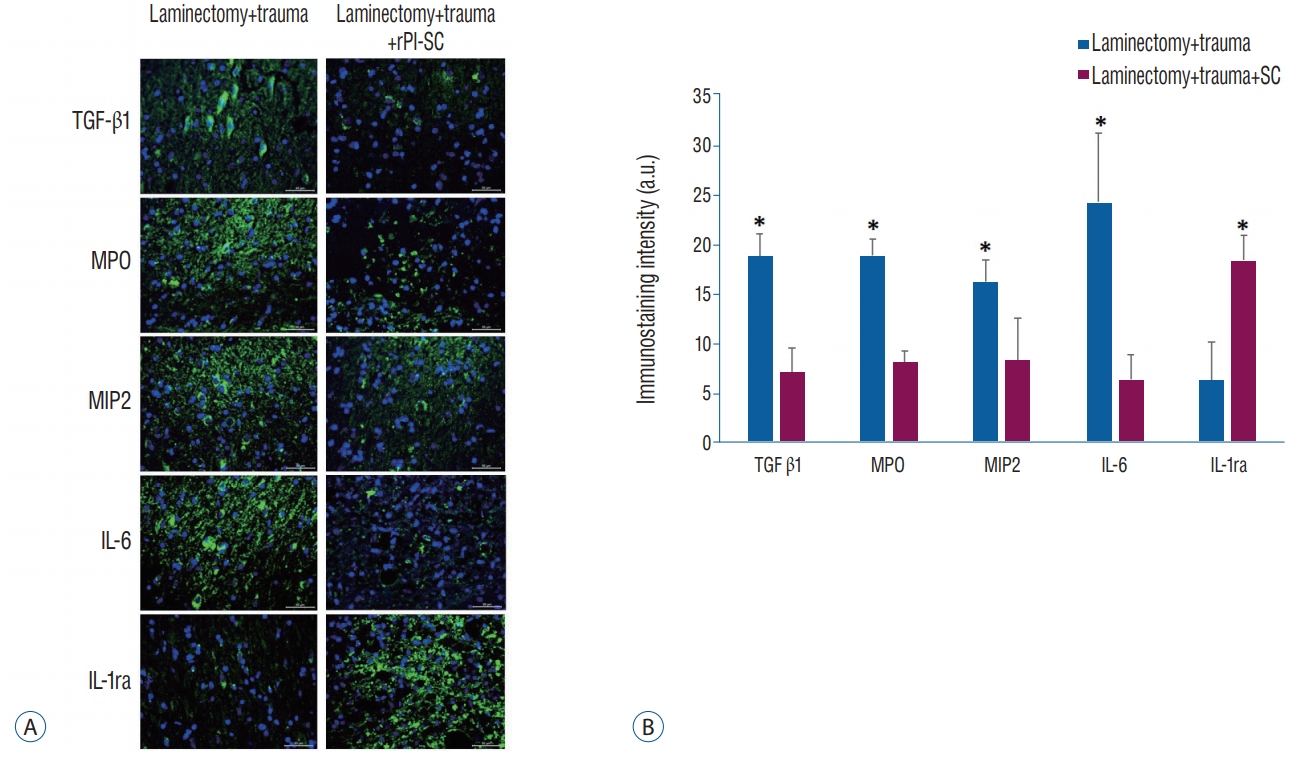
A : Immunofluorescence stainings for anti-inflammatory (IL-1ra) and pro-inflammatory (IL-6, TGF-β1, MIP-2, and MPO) markers with and without rPI-SC injection in paraffin sections of rat spinal cord. All markers were detected with FITC (green) labelled secondary antibodies. Nuclei were labeled with DAPI (blue). Scale bars is 50 µm. B : Graphs of immunostaining intensities. The values were presented as mean±standard error. *p<0.05. rPI-SC : rat pancreatic islet derived stem cell, TGF-β1 : transforming growth factor-1, MPO : myeloperoxidase, MIP-2 : macrophage inflammatory protein-2, IL-6 : interleukin-6, IL-1ra : interleukin-1 receptor antagonis, a.u. : arbitrary unit.
Functional recovery
To confirm the traumatic impact of the standardized severe weight-drop contusion injury to the T10–T11 spinal cord, the hind limb locomotion of the SCI rats were evaluated. At each assessing time point, consistent functional deficits were noted among SCI rats with the BBB locomotion scores showing profound loss initially, which was then gradually improved and approaching a plateau level of spontaneous recovery typical for this type of injury by 4 weeks p.i. (Fig. 9).
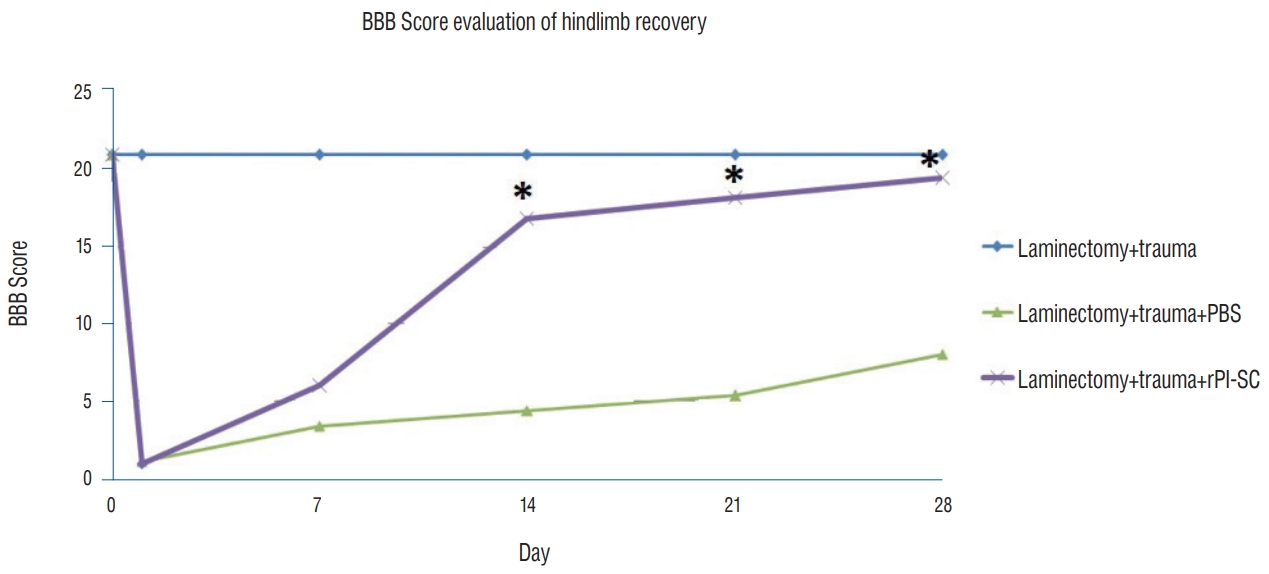
The effect of T10–T11 SCI on general hind limb function over time after SCI. Deficits are expressed as a BBB locomotion score. The values were presented as mean±standard error. *p<0.05. BBB : Basso, Beattie and Bresnahan, PBS : phosphate-buffered saline, rPI-SC : rat pancreatic islet derived stem cell, SCI : spinal cord injury.
According to BBB locomotor activity test, the performances of the laminectomy+trauma+SC group were statistically different from the laminectomy+trauma group (p<0.05). The injured rats showed marked lower activity score than MSC injected group in the BBB locomotor rating score. All experimental groups showed BBB locomotion score increment to some extent but the highest scores were observed in the stem cell injected group (Fig. 9). Hindlimb movements recorded by video camera at each assessing time points can be seen in Supplementary Video 1.
DISCUSSION
There has been considerable effort for healing SCI both with experimental models [1] and human phase 1 and 2 trials [48] based on stem/progenitor cell transplantation. SCI is known to have an ischemic basis, complicated by an acute inflammatory and edematous tissue response [17]. Thus, cell-based therapy has an important role in reducing these SCI related cellular symptoms.
In some models of SCI, MSCs are suggested to reduce tissue damage, decrease cyst and injury size and improve functional outcomes [22]. In our previous studies we have also shown the efficiency of bone marrow [27] and adipose tissue [4] MSCs on functional recovery following spinal cord trauma.
In the present study, we focused on PI-SCs, since pancreatic cells were reported to have regenerative capacity [26,44,45,59], suggested to result from multipotent stem cell characteristics [44,45]. In addition to giving rise to new pancreatic islet cells, PI-SCs were also shown to differentiate into neural cells with the capability of extensive proliferation and self-renewal [9,45].
Ontogenic origin of pancreas-derived multipotent precursors (PMPs) was considered to be the neural crest (NC) [42]. Post-migratory NC derived cells from different regions are able to generate neuronal, glial and non-neural cell types [57]. Our findings revealing that cells isolated from PI express mesenchymal markers (CD29, CD54, CD90, mhc class II, vimentin) (Figs. 1D, 2D) are consistent with previous reports [10]. Since, PMPs were derived from NC [42], rPI-SCs in this study might also be originated from NC and acquired MSC characteristics.
In this study it was also revealed that, cells isolated from pancreas express important components of nerve regeneration such as BDNF (Fig. 2A) and fibronectin (Fig. 2C). BDNF provides neuroplasticity, cell survival, axonal elongation, and neurite outgrowth through the activation of mitogen-activated protein kinase (MAPK), phospholipase C- (PLC- ), and phosphatidylinositol-3 kinase (PI3K) pathways [6]. Fibronectin is an extracellular matrix component known to be expressed in spinal cord [36] and fibronectin biomaterials have been developed for use in the repair of injured spinal cord [29]. Expression of these factors in PI cells might have a contribution in neural regeneration in the injured spinal cord.
As reviewed by Heit and Kim [21], pancreatic endocrine development was demonstrated to have numerous similarities with neural development. In the current study, rPI-SC was shown to express neural cell markers such as MAP2a,b (Fig. 2E), β3-tubulin (Fig. 2F) and nestin (Fig. 2G), suggesting that they have a potential for neural differentiation. Since, nestin positive pancreatic cells have been shown to carry stem/progenitor cells [63] and have neural differentiation potential [31], nestin positive cells in this study might be included in neural differentiation in the injured spinal cord. Additionally, neuroprotection and neurogeneration markers such as S100β (Fig. 5) and BDNF (Fig. 6), as well as oligodendrocyte marker CNPase (Fig. 4) were detected in GFP+ cells in the injury site. Isolated rPI-SC in the current study also showed GFAP expression (Fig. 2B), suggesting that these cells might differentiate into glial cells, since GFAP is an intermediate filament in mature astrocytes of the CNS [19].
Astroglial calcium-binding protein S100β is predominantly found in astroglial cells [18]. It has been suggested that S100β in CNS tissue is involved in neuroprotection and neuroregeneration [43]. Our results revealed that GFP labelled cells migrated to the injured area were S100β positive (Fig. 5), suggesting that these cells could have contribute to the healing of the injured spinal cord through S100β.
VEGF has a pivotal role in angiogenesis and neovascularization, cell migration, inducing proliferation, repressing apoptosis as a neurotrophic factor [41]. Since, GFP+ cells were also shown to express VEGF (Fig. 7A), we also suggest that, rPISCs might contribute to neuro-regeneration through VEGF signaling.
An important finding revealed in our investigation was that rPI-SCs could be effective in modulating inflammatory conditions. During the secondary injury cascade of tissue destruction process following SCI, infiltrated inflammatory cells induce the release of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1α, IL-1β and IL-6 [3,60]. Accordingly, control of inflammation holds promise for the improvement of SCI repair [55].
MSCs can modulate local and systemic inflammation after SCI where MSC treatment led to a decrease in peripheral inflammatory cell infiltration [28]. It was demonstrated that BMSCs seeded in scaffolds were able to decrease the distribution of blood-derived immune cells around the SCI to promote functional recovery [56]. In the current study, remarkable effect of rPI-SC transplantation on hindlimb movements of the rats after SCI (Fig. 9, Supplementary Video 1), might be through the regulation of inflammation factors.
Various studies demonstrated that MSC displayed their therapeutic benefits by paracrine regulation with growth factors and cytokines for promoting vascular repair in the disease-associated situation [2]. In the current study, after performing SCI, the injection of PI-SCs was likely to prevent immune cell activation and in particular to reduce the secretion of proinflammatory cytokines including IL-6, MIP-2, MPO, IL-1β and TGF-β (Fig. 8) as possible direct markers of the inflammation of spinal cord. Inhibition of these inflammation factors positively affects the healing process of SCI. For instance, application of IL-6 receptor monoclonal antibody decreased the number of inf lammatory cells and scar formation in mouse SCI models [37]. MSC-derived prostaglandin E2 was reported to act on macrophages, increasing their IL-10 secretion and reducing inf lammation [32]. These actions of prostaglandins take place after they bind to their G-protein-coupled receptors such as EP3 [52]. We demonstrated the presence of EP3 in rPI-SCs (Fig. 1I) which might be pointing out immunosuppressive activity of these cells.
In previous studies, rBM-MSC transplantation in rats was shown to consistently attenuate the levels of pro-inflammatory cytokines including IL-6 and MIP-2 [27] and MPO [61]. Németh et al. [34] demonstrated that anti-inflammatory mediators like IL-10, IL-1ra and IL-13 increased after MSC treatment. IL-1ra inhibits IL-1 that attracts neutrophils, macrophages, and lymphocytes resulting in tissue inflammation [53] and directly enhances epithelial cell survival [38]. In the current study, rPI-SC administration was found to be effective for increasing the intensity of IL-1ra (Fig. 8) in the injured area of the spinal cord, suggesting an anti-inflammatory role for these cells.
Since particular factors related to neutrophil accumulation, such as MPO and MIP were previously suggested to be related with inflammation after SCI [20], suppression of these factors is an essential therapeutic step for SCI. We have shown that these factors were decreased after stem cell injection in the injured area (Fig. 8). In addition, TGF-β, which has been suggested to be increased after SCI [16], was also decreased after rPI-SC injection. The altered levels of all these factors related to inflammation after rPI-SC transplantation, provide strong evidence that rPI-SCs might have an impact on modulation of anti-inflammatory response for healing SCI.
CONCLUSION
The present study showed that transplantation of rPI-SCs into the contused spinal cord improved locomotor recovery. Reduction of inflammation factors after rPI-SCs transplantation might be effective for functional outcomes following traumatic injuries to the spinal cord. In light of the findings of the current study, we suggest that rPI-SCs can be used as a unique cellular resource for neuroregeneration therapies. The use of post-transcriptional regulators might provide further improvement in rPI-SC based cellular therapy for SCI. Because of the convincing results of applications of rPI-SCs in experimental models for functional recovery after SCI, we suggest that PI-SCs might be considered for the use in clinical trials for their therapeutic use in the injuries of human spinal cord.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors would like to thank Alparslan Okcu and Asst Prof. Gokhan Duruksu for their technical assistance.
Supplementary Materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2018.0035.