Surgery versus Conservative Treatment for Spontaneous Supratentorial Intracerebral Hemorrhage in Spot Sign Positive Patients
Article information
Abstract
Objective
An advantage of surgical treatment over conservative treatment of spontaneous intracerebral hemorrhage (ICH) is controversial. Recent reports suggest that contrast extravasations on CT angiography (CTA) might serve as a crucial predictor of hematoma expansion and mortality. The purpose of this study was aimed at investigating the efficacy of surgical treatment in patients with spot sign positive ICH.
Methods
We used our institutional medical data search system to identify all adult patients who admitted for treatment of ICH between January 1, 2007 and January 31, 2012. Patients were classified two groups into a surgical group (n=27) and a conservative treatment group (n=28). Admission criteria were the following: age 20-79 years, spontaneous supratentorial ICH, Glasgow Coma Score Ranging from 9 to 14, ICH volume ≥20 mL, and treatment within 24 hours.
Results
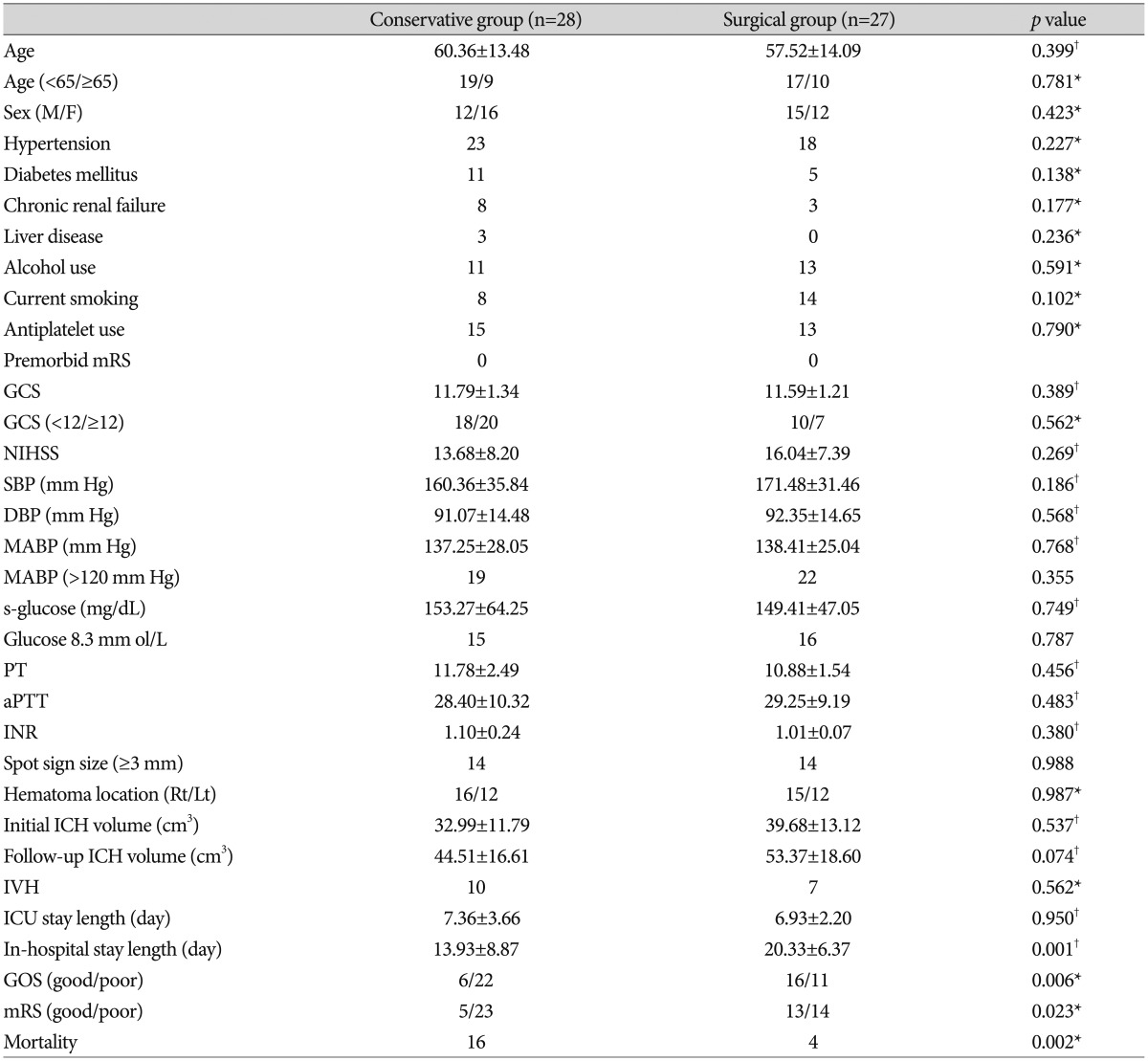
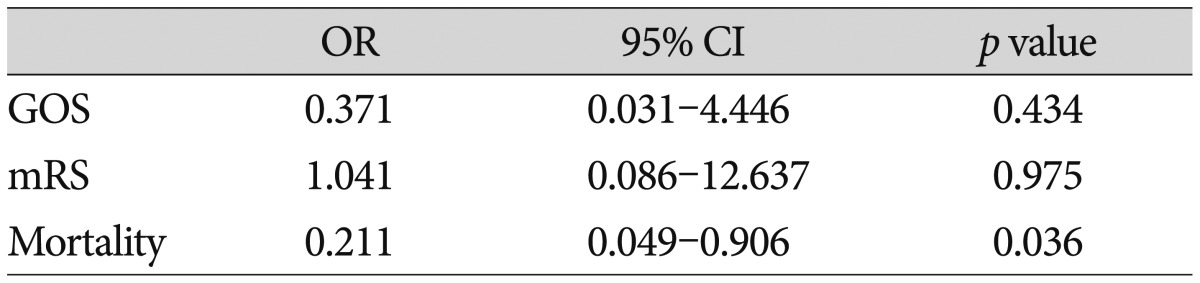
Fifty-five patients were analyzed. There was no significant difference in the ICU stay between the conservative treatment group (7.36±3.66 days) and the surgical treatment group (6.93±2.20 days; p=0.950). There was a significant difference in the in-hospital stay between the conservative treatment group (13.93±8.87 days) and the surgical treatment group (20.33±6.37 days; p=0.001). Overall mortality at day 90 after ICH was 36.4%; this included 16 of 28 patients (57.1%) in the conservative group and 4 of 27 patients (14.8%) in the surgical group. In univariate analysis, there was a positive effect of the surgical treatment in reducing mortality at 90 days (p=0.002), Glasgow Outcome Scale (GOS) at 90-day (p=0.006), and modified Rankin Scale (mRS) at 90-day (p=0.023). In multivariate logistic analysis, there was a significant difference in mortality (odds ratio, 0.211; 95% confidence interval, 0.049-0.906; p=0.036) between the groups at 90-day follow-up. However, there was no significant difference in GOS (odds ratio, 0.371; 95% confidence interval, 0.031-4.446; p=0.434) and mRS (odds ratio, 1.041; 95% confidence interval, 0.086-12.637; p=0.975) between the groups at 90-day follow-up.
Conclusion
In this study of surgical treatment of supratentorial ICH in patients with spot sign positive in CTA was associated with less mortality despite of long duration of in-hospital stay. We failed to show that clinical outcome benefit of surgical treatment compared with conservative treatment in patients with spot sign positive ICH.
INTRODUCTION
Intracerebral hemorrhage (ICH) is the most disabling form of stroke. ICH is subtype of stroke with high morbidity and mortality accounting for 10-17% of all deaths from stroke124). Approximately 40% of patients with intracerebral hemorrhage die within 30 days, and the majority of survivors are left with severe disability471217). Hematoma expansion has been identified as one of the most important determinants of early neurological deterioration and poor outcome in primary ICH717). The 30-day mortality is correlated with the size and location of the initial bleeding511). Deep hemorrhages are associated with high mortality rates. Recently, advanced surgical techniques, neuroanaesthesia, neuronavigation system, and improved perioperative management have led to improved outcomes from surgery in many conditions. There is no clear indication of the optimal treatment of these patients, surgical or otherwise. There are currently 6 published randomized trials that have studied the surgical evacuation of spontaneous ICH2321232731). All randomized trials are carefully analyzed; there is no net benefit from surgery. Recently, several studies suggested that contrast extravasations on CT angiography (CTA) might serve as a crucial predictor of hematoma expansion and mortality891329). The presence of active contrast extravasations into the hematoma at the time of multidetector CT angiography (MDCTA), the spot sign, is an indicator of active hemorrhage and has been associated with an increased risk of hematoma expansion and mortality in patients with ICH7891329). Proper selection of patients at high risk for hematoma expansion seems crucial to improve outcomes. The purpose of this study is to review retrospective patients with spot sign positive ICH to assess whether surgical evacuation of the hematoma would improve outcome, in terms of death and disability, compared with conservative treatment.
MATERIALS AND METHODS
We used our institutional medical data search system to identify all adult patients who admitted for treatment of ICH between January 1 2007 and January 31, 2012. We conducted a retrospective review of all consecutive patients who admitted to the department of neurosurgery. To be eligible for the study, patients with ICH need to meet the following inclusion criteria : 1) evidence of nontraumatic ICH on a noncontrast CT examination of the head, 2) evaluation with a CTA of the intracranial circulation within 24 hours of presentation, 3) only patients with spot sign positive in MDCTA, 4) ICH volume is over 20 mL, and 5) age between 20 and 80 years. The exclusion criteria were : 1) patients with poor or good neurologic status [Glasgow Coma Scale (GCS) 3-5, or 15], 2) brain stem hemorrhage and cerebellar hemorrhage, 3) a previous stroke history with neurological deficits, 4) pure intraventricular hemorrhage (IVH), 5) secondary intracerebral hemorrhage such as arteriovenous malformation, moyamoya disease, tumor bleeding, and venous sinus thrombosis, 6) incompletion of a standard CT protocol including noncontrast CT (NCCT) and MDCTA, 7) cortical hemorrhage, and 8) refuse surgical treatment despite of surgical indication.
Clinical data
Clinical data of patients with ICH were collected by neurosurgeon blinded to the radiological data and at the 90-day follow-up. The collected demographic and clinical variables included sex, age, alcohol, and smoking use, history of hypertension, diabetes mellitus, chronic renal failure, liver disease (liver cirrhosis and hepatocellular carcinoma), and medications (antihypertensive and antiplatelet agents). The systolic, diastolic, and mean arterial blood pressure of patients was recorded. Stroke severity on admission was evaluated by GCS and National Institutes of Health Stroke Scale (NIHSS). Laboratory tests on admission included serum glucoses, activated partial thromboplastin time, and prothrombin time as expressed by the international normalized ratio. The patients' clinical outcome was assessed by Glasgow Outcome Scale (GOS) and modified Rankin Scale (mRS) on discharge and 90-day follow-up. Poor clinical outcome was defined as GOS <4 and mRS >2. Intensive care unit (ICU) length of stay and in-hospital length of stay was recorded. The variables subgroups were : age ≥65 vs. <65 years); hematoma volume (≥40 mL vs. <40 mL); GCS (9 to 12 vs. ≥13); or antiplatelet treatment (any vs. none).
Radiological data
NCCT and MDCTA acquisitions were performed according to standard departmental protocols on 64-section General Electric helical CT scanners (Lightspeed; GE Medical Systems, Waukesha, WI, USA). Imaging was performed as follows : 1) initial and 24-hour follow-up NCCT scans were performed using 4.5 mm contiguous axial sections from skull base to vertex parallel to the inferior orbitomeatal line. Hematoma location was classified as right or left. And the presence of IVH was recorded. Determination of the initial and follow-up ICH volumes was performed independently and blinded to the initial NCCT and follow-up CT. The reviewer was measure the volume of hemorrhage in milliliters by using the ABC/2 method, where A is the greatest diameter of hemorrhage on the CT section with the largest area of hemorrhage, B is the diameter perpendicular (90°) to A, and C is the number of sections with hemorrhage multiplied by the section thickness16). C was calculated by a comparison of each CT section with hemorrhage with the CT section demonstrating the largest area of hemorrhage on that scan. The spot sign was defined according to four criteria : 1) serpiginous or spot-like appearance within the margin of a parenchymal hematoma without connection to an outside vessel, 2) contrast density greater than 1.5 mm in diameter in at least one dimension, 3) contrast density (Hounsfield units, HU) at least double that of the background hematoma, and 4) no hyperdensity at the corresponding location on non-contrast CT (Fig. 1)28). An increase of hematoma volume >33% or >12.5 mL was considered as hematoma expansion51315).
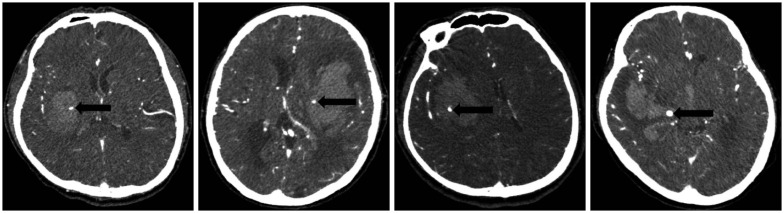
The appearance of a spot sign on CT angiography in a patient with intracerebral hemorrhage. The spot sign (black arrows) is located within the hematoma, has no connection to any outside vessel, and is absent on baseline non-contrast CT.
The surgical approach was individualized on the basis of the site and size of the ICH because of the lack of standardized guidelines for allocation of operative treatment. Allowed techniques included open craniotomy and neuronavigation-guided placement of a catheter for evacuation of supratentorial ICH. The intention of surgical treatment was complete removal of the clot. Surgery was performed in patients with impending cerebral herniation, as indicated by abnormal pupil response, abnormal posture, or CT findings of absence of ambient cistern or severe midline shifting (>5 mm). Surgery was performed at least 24 hours after onset in all cases.
Stereotactic aspiration of the hematomas was performed in the acute phase between 6 to 24 hours after onset of stroke. Patients underwent repeat CT scan with a stereotactic protocol that utilized 1.5 mm slices that were then loaded onto a Stealth Station (Medtronic, Inc., Minneapolis, MN, USA). Under general anesthesia, the patient's head was fixed in a Mayfield clamp and a reference arc was attached to the apparatus. Hematoma was continuously liquefied by urokinase (containing 50000 U urokinase/2-3 mL saline solution) for 2-4 days (2-3 times per day).
Patients in the surgical and medical group received standard supportive medical care in the neurological center where they had been initially admitted. Therapy included blood pressure control, intravenous fluids, hyperosmolar agents, H2 blockers, maintenance of normoglycemia, early nutritional support, and physical therapy. Therapy included pneumatic compression boots for deep vein thrombosis prophylaxis, physical therapy, intravenous fluids, H2 blockers, maintenance of normoglycemia, and early nutritional support. Intubation was performed in patients as needed for respiratory depression, airway protection, and/or control of intracranial pressure (ICP). Hyperventilation and steroids were discouraged, but their use by an individual physician did not exclude patients from the study. We did not use any hemostatic agents. Hypertension was regulated early in the course of therapy. Systolic arterial blood pressure (SABP) was maintained between 120 and 140 mm Hg by appropriate antihypertensive therapy intravenous treatment and therapy with oral agents were to be initiated according to treatment protocols that were based on the local availability of agents, with the goal of achieving a systolic blood pressure level of less than 140 mm Hg within 1 hour after admission and of maintaining this level for the next 7 days.
Treatment included invasive monitoring of ICP when indicated. Intracranial pressure measurement was not a standard procedure in our protocol. In the presence of an ICP monitor, cerebral perfusion pressure (MAP2ICP) was maintained at 70 to 100 mm Hg.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences for Microsoft Windows (Version 12.0; SPSS, Inc., Chicago, IL, USA). Patients were classified according to survival versus dead and good versus poor clinical outcome (GOS 4-5 vs. 1-3 and mRS 0-2 vs. 3-6) on discharge and at 90-day follow-up. The associations of surgical treatment with clinical outcomes and mortality were examined by the chisquare test for categorical variables or Mann-Whitney U-test for continuous variables. Univariate analyses were performed for association of demographic, clinical, laboratory, and imaging variables with respect to surgical treatment. Categorical variables were compared between groups with the chi-square test for significance. The Mann-Whitney U test was used for continuous variables. The multivariate logistic regression analysis was repeated for the prediction of surgical treatment. Adjusted odds ratios (OR) and their 95% confidence intervals (95% CI) were calculated. Significance was set at a p value of less than 0.05.
RESULTS
There were 55 patients diagnosed with primary ICH fulfilled the study inclusion criteria, and analyzed. All patients had spot sign in MDCTA. Surgery included open craniotomy and stereotactic hematoma aspiration, but excluded extraventricular drainage. Thirty-one hematomas (56.4%) were located on the right side, whereas twenty-four hematomas (43.6%) were located on the left side. Of the 41 patients (74.5%) with ICH for whom a history of hypertension was confirmed, elevated blood pressure levels were recorded in 53 (93%). The surgical and the medical groups were comparable with respect to baseline characteristics (age, sex, diabetes mellitus, NIHSS score, GCS score, blood pressure, glucose, coagulation profiles, hematoma volume, outcome, mortality, ICU stay, and in-hospital stay) (Table 1). Twenty-seven patients were surgical group, and twenty-eight patients were medical group. Univariate analyses demonstrated the age, sex, hypertension, diabetes mellitus, chronic renal failure, liver disease, alcohol, smoking, antiplatelet agents, GCS, GCS (<12), SBP, DBP, MABP, MABP (>120 mm Hg), PT/aPTT, INR, and glucose ≥8.3 mmol/L had no significant association between groups. The follow-up CT was checked between 3-10 hours after initial CT. In our study, 15 patients received operation between 7-10 hours after onset, and hematoma expansion occurred in 10 (66.7%). 6 patients received operation between 3-6 hours after onset, hematoma expansion occurred in 3 (50%). And 6 patients received operation between 10-24 hours after onset, hematoma expansion occurred in 3 (50%). The mean NIHSS score was 13.68 in the conservative treatment group and 16.04 in the surgical treatment group (p=0.269). The NIHSS in surgical group is slightly higher than medical group, but there is no significant difference between groups. The mean GCS score was 11.79 in the conservative treatment group and 11.59 in the surgical treatment group (p=0.389). The large spot sign (≥3 mm) was recorded 14 patients in conservative treatment group, and 14 patients in surgical treatment group (p=0.988). The mean initial ICH volume was 32.99 cm3 in the medical treatment group and 39.68 cm3 in the surgical treatment group (p=0.537). The mean follow-up ICH volume was 44.57 cm3 in the medical treatment group and 53.37 cm3 in the surgical treatment group. The follow-up ICH volume was smaller in the conservative treatment group, but there was no significant difference (p=0.074). ICU length and in-hospital length of stay and was analyzed. There was no significant difference in the ICU stay between the conservative treatment group (7.36±3.66 days) and the surgical treatment group (6.93±2.20 days; p=0.950). However, there was a significant difference in the in-hospital stay between the conservative treatment group (13.93±8.87 days) and the surgical treatment group (20.33±6.37 days; p=0.001). This difference is due to early death in conservative treatment group resulted in a shortened inpatient stay. Patients were classified according to good versus poor clinical outcome (mRS 0-2 vs. 3-6, GOS 4-5 vs. 1-3) on discharge and at 90-day follow-up. The mortality at day 90 after hemorrhage was 20 patients (36.4%). This included 16 of 28 patients (57.1%) in the conservative treatment group and 4 of 27 (14.8%) in the surgical treatment group (p=0.002). In univariate analysis, there was a positive effect of the surgical treatment in reducing mortality at 90 days (p=0.002), GOS at 90-day (p=0.006), and mRS at 90-day (p=0.023).
In multivariate logistic analysis, there was a significant difference in mortality (odds ratio, 0.211; 95% confidence interval, 0.049-0.906; p=0.036) between the groups at 90-day follow-up (Table 2). However, there was no difference in GOS (odds ratio, 0.371; 95% confidence interval, 0.031-4.446; p=0.434) and mRS (odds ratio, 1.041; 95% confidence interval, 0.086-12.637; p=0.975) between the groups at 90-day follow-up (Table 2). In this series, outcome at 90 day follow-up measures showed no significant trend toward a better outcome in the surgical group than the medical treatment group for GOS and mRS.
DISCUSSION
In our series, we show that mortality at 90 day follow-up in patients with spot sign positive ICH treated with conservative treatment versus surgery do differ significantly, but clinical outcomes do not differ significantly. Controversy continues to surround the appropriate treatment of ICH.
Hematoma volume is a critical determinant of outcome in ICH, especially if measured early after the onset of symptoms457131725). Hematoma expansion is now recognized to occur to a clinically important degree in 70% of cases of ICH within the first 24 hours after the onset of symptoms; such growth is associated with increased mortality and poor functional outcome726). Therapeutic options to restrict hematoma expansion can be divided into surgical and nonsurgical approaches. Many surgical trials failed to show an outcome benefit over conservative treatment314212331). And some medical treatment with recombinant activated factor VII (rFVIIa) limits the growth of hemorrhage, reduces mortality, and improves functional outcomes after ICH20). Batjer et al.3) showed that current medical and neurosurgical therapies remain ineffective in preventing the devastating neurologic consequences of hypertensive putaminal hemorrhage3). Report from the Surgical Trial in Intracerebral Haemorrhage (STICH) trial showed no overall benefit of early surgical clot evacuation compared with initial conservative treatment in patients with ICH21). In our series, there was a positive effect of the surgical treatment in reducing mortality at 90 days (p=0.002), GOS (p=0.006), and mRS (p=0.023) in univariate analysis. In multivariate analysis, present study show significant difference in mortality and we failed to show that clinical outcome benefit of surgical treatment compared with conservative treatment in patients with spot sign positive ICH. In our series, patients in the initial conservative treatment group who later had surgery also did not have good outcomes. The timing of treatment also had a powerful effect on the treatment modality to limit the hematoma expansion because active bleeding occurs in a large proportion of patients with ICH within the first few hours after onset and rapidly diminishes over time1820). Morgenstern et al.23) performed studies to answer the intervention timing. Indeed, the first study revealed better outcome for those patients operated within 12 hours after onset. However, the follow-up study with a time window of 4 hours showed a higher mortality due to an increased rebleeding rate22). Various timing of surgical treatment may influence on the clinical outcome. This is most weak point in our series. Recently, alternative surgical procedures include endoscopic surgery and stereotactic aspirations are widely performed procedure62730). There was a significant difference in the ICU stay between the conservatively treated group (1.8 days, SD 7.1 days) and the surgical group (3.9 days, SD 6.1 days; p=0.007)27). In our series, there was a significant difference in the in-hospital stay between the conservative treatment group (13.93 days, SD 8.8 days) and the surgical treatment group (20.33 days, SD 6.3 days; p=0.001), in spite of no significant difference in ICH stay (7.36 vs. 6.93 days; p=0.950). This difference is due to early death in conservative treatment group resulted in a shortened inpatient stay. In mortality group, ICU stay was 9.1 days, and in-hospital stay was 13.0 days. And survival group, ICU stay was 6.0 days, and in-hospital stay was 19.3 days. These suggest that mortality group is stay longer in ICU, and shorter in-hospital than survival group. Consequently, there was a significant difference in the in-hospital stay between the conservative treatment group and the surgical treatment group. Several studies reported that therapeutic options to restrict hematoma expansion can be medical treatment. The large randomized controlled trial addressing the effect of lowering blood pressure in ICH has been published and conclude that early intensive BP-lowering (target SBP 140 mm Hg) treatment is clinically feasible, well tolerated, and seems to reduce hematoma growth in ICH1).
Early restriction of ICH is of paramount importance because secondary volume expansion leads to outcome deterioration and death26). Recent studies suggested that the most powerful predictor of hematoma expansion and poor clinical outcomes is spot sign on CTA8101319). Contrast extravasations so called spot sign on admission CT angiography as a predictor of hematoma expansion was also found in an additional retrospective study, recently extended to a proposed spot sign score, and prospective study81319). Most recently, Demchuk et al.10) concluded that a subpopulation of patients with ICH with the spot sign who are at high risk of substantial intracerebral and intraventricular hematoma expansion, early neurological deterioration, and early mortality10). A subpopulation of ICH in patients with spot sign positive is high risk mortality and poor clinical outcome81013). In this high risk group, the major goal of surgical treatment in patients with an ICH is safe and thorough hematoma evacuation with maximal preservation of neurological function. We retrospectively analyzed the effect of surgical treatment in patients with spot sign positive ICH. In our series, we included patients with GCS >5.
The mortality rate at 3 months was 14% in the surgery group, and 57% in the conservative group (Table 1). There was difference in mortality between the two groups (Table 2). There were differences in the causes of death between the two groups. Patients in the surgery group died from cardiac events [2 (50%) of 4], pulmonary infection [1 (25%) of 4], unknown reason [1 (25%) of 4]. Patients in the conservative group died from respiratory infections [4 (25%) of 16], cardiac events [3 (18%) of 16], renal failure [3 (18%) of 16], and unknown reason [6 (38%)of 16]. The significant difference in mortality between two groups may be related to the discrepancy of underlying diseases (diabetics mellitus, chronic renal failure, liver disease) (Table 1), and short in-hospital stay in conservative groups. The possible explanations of the difference in mortality between two groups are not only surgical effectiveness but also discrepancy of underlying diseases. Despite of no significant difference of underlying disease in two groups, discrepancy of underlying diseases has the important role in mortality. We showed that surgical treatment reduced mortality, but the quality of life remains poor despite of relative good neurological status. Possible explanations for these findings are that deep seated ICH may destruct internal capsule, and surgical treatment could not convert neurologic deficits. Our findings highlight an important incremental benefit of surgical treatment in patients with spot sign positive ICH, surgical treatment in patients with spot sign positive ICH are far less likely to result in death. Future prospective studies include the combination of surgical and medical treatment. Surgical treatments include conventional craniotomy, minimally invasive stereotactic hematoma aspiration, and endoscopic hematoma removal. Medical treatments include rFVIIa and neuroprotectants despite of failure in ischemic stroke. A randomized controlled trial of surgical treatment versus conservative treatment in patients with a spot sign positive ICH would be necessary to compare differences in mortality and clinical outcomes.
Although the surgical and medical groups were relatively well balanced with regard to several baseline variables that are related to outcome (such as age, NIHSS and GCS scores, and ICH volume), This study's limitations are its retrospective design, and the patients with GCS 3 to 8 were excluded. And selective basis influences in results. The sample size of 55 patients is small. In our study, the timing of surgical treatment are various because of unintended study. A prospective study with larger number of cases is needed to confirm the association of surgery to favorable clinical outcomes and reducing mortality in patients with spot sign positive ICH.
CONCLUSION
In this study of surgical treatment of supratentorial ICH in patients with spot sign positive in CTA was associated with less mortality despite of long duration of in-hospital stay. We failed to show that clinical outcome benefit of surgical treatment compared with conservative treatment in patients with spot sign positive ICH. Proper evaluation of the role of surgery following spontaneous ICH in patients with spot sign positive should be undertaken in the format of a prospective randomized controlled trial. And future prospective studies include the combination of surgical and medical treatment.
Acknowledgements
The authors thank Professor Shin Jung, Department of Neurosurgery, Chonnam National University of Gwangju, Korea, for his useful remarks regarding the manuscript.
This paper was supported by research funds of Chonbuk National University in 2012.