Radiation Therapy against Pediatric Malignant Central Nervous System Tumors : Embryonal Tumors and Proton Beam Therapy
Article information
Abstract
Radiation therapy is highly effective for the management of pediatric malignant central nervous system (CNS) tumors including embryonal tumors. With the increment of long-term survivors from malignant CNS tumors, the radiation-related toxicities have become a major concern and we need to improve the treatment strategies to reduce the late complications without compromising the treatment outcomes. One of such strategies is to reduce the radiation dose to craniospinal axis or radiation volume and to avoid or defer radiation therapy until after the age of three. Another strategy is using particle beam therapy such as proton beams instead of photon beams. Proton beams have distinct physiologic advantages over photon beams and greater precision in radiation delivery to the tumor while preserving the surrounding healthy tissues. In this review, I provide the treatment principles of pediatric CNS embryonal tumors and the strategic improvements of radiation therapy to reduce treatment-related late toxicities, and finally introduce the increasing availability of proton beam therapy for pediatric CNS embryonal tumors compared with photon beam therapy.
INTRODUCTION
In recent decades, radiation therapy has changed greatly through the development of radiation delivery and treatment planning systems. Like other cancer treatment modalities, the main purposes of radiation therapy are to maximize the local tumor control and minimize the treatment-related late toxicities. Especially for children, radiation therapy should be carefully applied because the radiation tolerance of children is much lower than that of adult patients and the late effect of radiation will last forever.
Among the pediatric solid tumors, central nervous system (CNS) embryonal tumors are medulloblastomas, supratentorial primitive neuroectodermal tumors (CNS embryonal tumour, not otherwise specified [NOS] in 2016 World Health Organization [WHO] classification) and atypical teratoid/rhabdoid tumors (AT/RTs). The basic principle of treatment for CNS embryonal tumors is maximal tumor resection followed by radiation therapy and chemotherapy. However, CNS embryonal tumors can be most strongly affected by radiation because the incidence is highest in young children aged 1–4 years and radiation therapy targeting whole neuroaxis is one of the standards of treatments.
To reduce the treatment-related late toxicities without compromising the outcomes, treatment strategies have developed directing to intensification of chemotherapy and decrease of radiation dose or irradiated volume. In addition, the introduction of particle beams such as proton beam therapy for pediatric tumors has increased during the last decade.
In this review, the principles of managements for pediatric CNS embryonal tumors and the strategic developments to reduce treatment-related late toxicities are provided, and the proton beam therapy for CNS embryonal tumors is introduced and compared with photon beam therapy.
CNS EMBRYONAL TUMORS AND TREATMENT
CNS tumors are the most common types of solid tumors in children and it accounts for 20–25% of all pediatric malignancies. CNS embryonal tumors constitute about 20% of all pediatric CNS tumors and medulloblastoma is the most common type of CNS embryonal tumors. Therefore, medulloblastoma is the most common pediatric malignant CNS tumor and it represents 15–20% of pediatric CNS tumors and 40% of posterior fossa tumors [8].
Medulloblastoma patients are stratified into standard-risk or high-risk group according to the age at presentation, the presence of anaplasia, the extent of postoperative residual tumor and the presence of tumor dissemination into the cerebrospinal fluid (CSF) [20]. Standard-risk of medulloblastoma is defined by postoperative minimal residual tumor (<1.5 cm2), an age of 3 years or older, no anaplasia, and no evidence of tumor dissemination into the CSF. Likewise, high-risk medulloblastoma is defined by postoperative residual tumor more than 1.5 cm2 or an age of less than 3 years old or presence of anaplasia, or tumor dissemination into the CSF.
Supratentorial primitive neuroectodermal tumors (CNS embryonal tumour, NOS in 2016 WHO classification) have a similar biology to high-risk medulloblastoma and are considered as high-risk embryonal tumors. AT/RTs typically present in very young children less than 2 years old [28] and are diagnosed on the basis of a characteristic immunohistochemical lack of nuclear INI1 protein expression. They are associated with a high risk of early relapse and a very poor prognosis and also considered as high-risk embryonal tumors.
The treatment of pediatric malignant CNS tumors is still challenging because it requires a multidisciplinary approach and the treatment principles are maximal tumor resection followed by radiation therapy and chemotherapy. However, the radiation dose or volume and chemotherapeutic agents or dose intensification are modified according to the risk of tumor.
Standard-risk medulloblastoma patients were initially treated with 36 gray (Gy) of craniospinal irradiation (CSI) and posterior fossa (PF) boost up to 54 Gy with a daily dose of 1.8 Gy over 6 weeks in postoperative setting. However, the CSI dose reduction studies revealed that CSI dose would be reduced 23.4 Gy from 36 Gy without compromising treatment outcomes [18,27].
The early studies for adjuvant chemotherapy have failed to show the additional effect on postoperative radiation therapy alone, but the current recommendations of chemotherapy for patients with standard-risk medulloblastoma are concurrent radiochemotherapy using weekly vincristine and approximately one year of maintenance chemotherapy consisting of cisplatin, lomustine (cyclonexyl-chloroethyl-nitrosourea), and vincristine. There have been several studies with neoadjuvant chemotherapy prior to irradiation, but the delays in the initiation of radiation therapy have been associated with inferior outcomes.
Radiation therapy for high-risk medulloblastoma in children 3 years of age and older is 36 Gy of CSI with a boost to both PF and metastatic sites to 55.8 Gy. For chemotherapy, the ideal chemotherapeutic regimens have not been identified yet. However, the treatment results for high-risk medulloblatoma are disappointing and 5-year survival is less than 55% [7,25,30]. Therefore, to overcome the unsatisfactory treatment results, the intensification of chemotherapy using high-dose chemotherapy and autologous stem cell transplantation (HDCT/autoSCT) has been tried and shown some clinical benefit in children with highrisk or recurrent solid tumors [9,11]. With this scheme of managements using HDCT/autoSCT and reduced-dose of CSI (23.4 Gy or 30.6 Gy instead 36 Gy), Korean researchers showed 70% of 5-year event-free survival (EFS) in high-risk medullblastoma patients [23].
For high-risk CNS embryonal tumors developing in children younger than 3 years old, the neurocognitive outcomes are very poor after radiation therapy including CSI. Therefore, avoiding or deferring radiation therapy until after the age of 3 has been issued and the intensified chemotherapy strategies, which were the addition of systemic or intraventricular methotrexate to postoperative induction chemotherapy or the use of HDCT/autoSCT, have been investigated. Those studies showed a satisfactory outcome in some populations of children but resulted in early local recurrence. In recurrent cases, salvage radiation therapy showed tumor response, and some studies applying the early local radiation therapy instead CSI even in children less than 3 years old have been investigated to avoid the toxicities of CSI as well as reduce early local recurrences [21,26].
RADIATION THERAPY FOR CNS EMBRYONAL TUMORS
At the time of diagnosis, about 10–40% of medulloblastoma patients develop leptomeningeal seeding and it can metastasize extracranially such as bone. Because of high risk of dissemination to CSF, CSI is the major radiation therapy strategy for CNS embryonal tumors. After CSI, the boost irradiation to PF (or primary tumor bed) or metastatic sites should be followed. Historically, the standard radiation dose is 54–55.8 Gy including 36 Gy for craniospinal axis and 18–19.8 Gy boost for PF or primary tumor bed. However, during the last decades, the results of many clinical studies showed that it was possible to reduce CSI dose or radiation volume and to avoid or defer radiation therapy until after the age of 3 while maintaining or improving survival rates and decreasing radiation-induced late toxicities.
CSI dose reduction
In a phase III randomized trial by Pediatric Oncology Group and Children's Oncology Group (COG) [27], a reduced-dose of CSI (23.4 Gy) was compared with a standard-dose of CSI (36 Gy) and the conclusion was that reduced-dose neuraxis irradiation was associated with increased risk of early neuraxis relapse, and lower 5-year EFS and overall survival (OS) than the standard-dose of irradiation. However, after the subsequent study [18] which demonstrated that the addition of chemotherapy to reduced-dose of CSI prolonged survival, the reduced-dose of CSI (23.4 Gy) strategy is now considered as the standard radiation for standard-risk medulloblastoma patients. For high-risk medulloblastoma, there was a study trying to decrease CSI dose with the intensification of chemotherapy [1], but 36 Gy of CSI dose remains as the standard for high-risk medulloblastoma.
According to COG study that investigated the intellectual outcomes after the reduction of CSI dose from 36 Gy to 23.4 Gy, the decline in intelligence quotient was still substantial and the amounts of decline were 4.3 points per year. However, some degree of intellectual preservation was observed when compared with those associated with conventional radiation doses [22]. Furthermore, a subsequent CSI dose-reduction study showed that a reduced CSI dose of 18.0–23.4 Gy resulted in stable cognitive outcomes at 5 years after treatment [14]. Therefore, further CSI dose reduction trials continue to be investigated.
COG ACNS 0331 was a randomized study to determine whether reducing the CSI dose from 23.4 Gy to 18 Gy in young children aged 3 to 7 years dose not compromise EFS and OS (Fig. 1). The final results are not published, however, reduced dose of craniospinal axis irradiation was associated with higher event rates and worse survival. Therefore, 23.4 Gy of CSI is the standard radiation dose for standard-risk medulloblastoma.

COG ACNS 0331 study diagram for standard-risk medulloblastoma. For children aged 3 to 7 years, CSI dose (23.5 Gy vs. 18.0 Gy) and boost radiation volume (primary site only versus entire posterior fossa) are randomized (A). For children aged 8 years or more, only boost radiation volume is randomized (B). COG : Children's Oncology Group, CSI : craniospinal irradiation, PF : posterior fossa, VCR : vincristine, CDDP : cisplatin, CCNU : lomustine, CPM : cyclophosphamide.
Reduction of radiation volume
Despite neuroaxis dose reduction, the cognitive function decline continues to be a significant treatment-related toxicity and new radiation targeting guidelines to limit the boost volume to the primary site only was suggested. Several studies have demonstrated that the PF boost volume may be safely reduced to the primary tumor bed, allowing for preservation of normal organs such as the temporal lobes, hypothalamus, and cochleae [13,29].
COG ACNS 0331 study was a randomized study to determine if reducing the irradiated volume of the primary site tumor boost from the whole posterior fossa to the tumor bed only will not compromise EFS and OS regardless of age (Fig. 1). Survival rates following reduced radiation boost volumes were comparable to standard treatment volumes for the primary tumor site and radiation oncologist can adopt smaller boost volume for posterior fossa irradiation.
Avoidance or deferral of radiation therapy for young children less than 3 years old
CNS embryonal tumors in young children are associated with worse survival than in older children, because it is not easy to avoid disease progression with a limited use of radiation related with functional impairment of developing brain in younger ages. Therefore, treatment strategy using HDCT/autoSCT without radiation therapy or with deferring radiation therapy have been tried and showed positive results in highrisk or recurrent brain tumors. The recent studies suggested that further dose intensification of HDCT/autoSCT might improve the outcomes.
Some clinical trials using intensified chemotherapy in infants and young children with malignant CNS tumors made it possible to avoid or defer radiation therapy without negatively affecting survival rates [2,5,10,24]. However, in case of AT/RT, disease progression developed early in the course of treatment and radiation therapy might be more efficacious than chemotherapy, even for very young children [21,26]. Therefore, up-front radiation therapy is typically used early after surgery and can vary from localized therapy to CSI, depending on the patient’s age and the extent of disease.
PROTON BEAM THERAPY VERSUS PHOTON BEAM THERAPY
During radiation therapy, radiation exposure to normal brain surrounding the tumor is inevitable and it can lead to late toxicities and affect the quality of life for long-term survivors. The possible treatment-related sequelae after CNS irradiation include neurocognitive impairment, endocrine dysfunction, growth abnormality, and secondary malignancies [4,6,15-17,19]. The Children Cancer Survivor Study revealed that survivors of childhood cancer have a high rate of illness due to chronic health conditions from treatment and the risk of long-term morbidities was threefold higher among adults treated with cranial irradiation for CNS tumors in childhood than in their siblings without cranial irradiation [17]. Therefore, the development of radiotherapeutic modality or technique that can preserve more normal brain is one of the most considerable points for radiation oncologist. Especially for CNS embryonal tumors, it became more important because the entire craniospinal axis should be irradiated.
Photon (x-ray) beam radiation therapy is the conventional radiotherapeutic approach for pediatric tumors. High energy photon beams penetrate tissues and are targeted to the tumor to deliver therapeutic radiation dose. After the introduction of the advanced radiation delivery techniques such as three-dimensional conformal radiation therapy and intensity-modulated radiation therapy, the precision of radiation therapy has improved and radiation oncologist can deliver high dose of radiation to the tumor with minimizing the irradiated volume of normal tissues. However, the delivery of high dose to the tumor from multiple photon beams induces unwanted exposure of normal tissues to low to intermediate radiation dose, causing radiation-induced toxicities.
Otherwise, proton beam is basically distinct from photon beam. Charged particles such as proton make radiation oncologists handle the radiation more precisely and preserve normal tissues without compromising the radiation dose to the tumor. This is possible because of the physical property of proton beam named “Bragg peak” and the majority of proton’s energy is released within a few millimeters of Bragg peak. Before the Bragg peak, proton loses a small amount of energy, so delivers a low ‘entrance’ dose. Beyond the Bragg peak, proton has no energy, so delivers no ‘exit’ dose (Fig. 2). The physical advantages of proton beam over photon beam can reduce radiation-induced toxicities and improve quality of life for patients who become long-term survivors of certain pediatric CNS tumors.

Relative dose of proton and photon beams according to depth. The majority of proton’s energy is released within a few millimeters of Bragg peak (black arrow).
Fig. 3 shows the difference of delivered radiation dose from proton or photon beams when the whole spinal axis is irradiated for medulloblastoma patients. Proton beam has no exit dose beyond Bragg peak and there is little radiation to normal organs such as heart, liver, bowels which are located at the front of the radiation target.
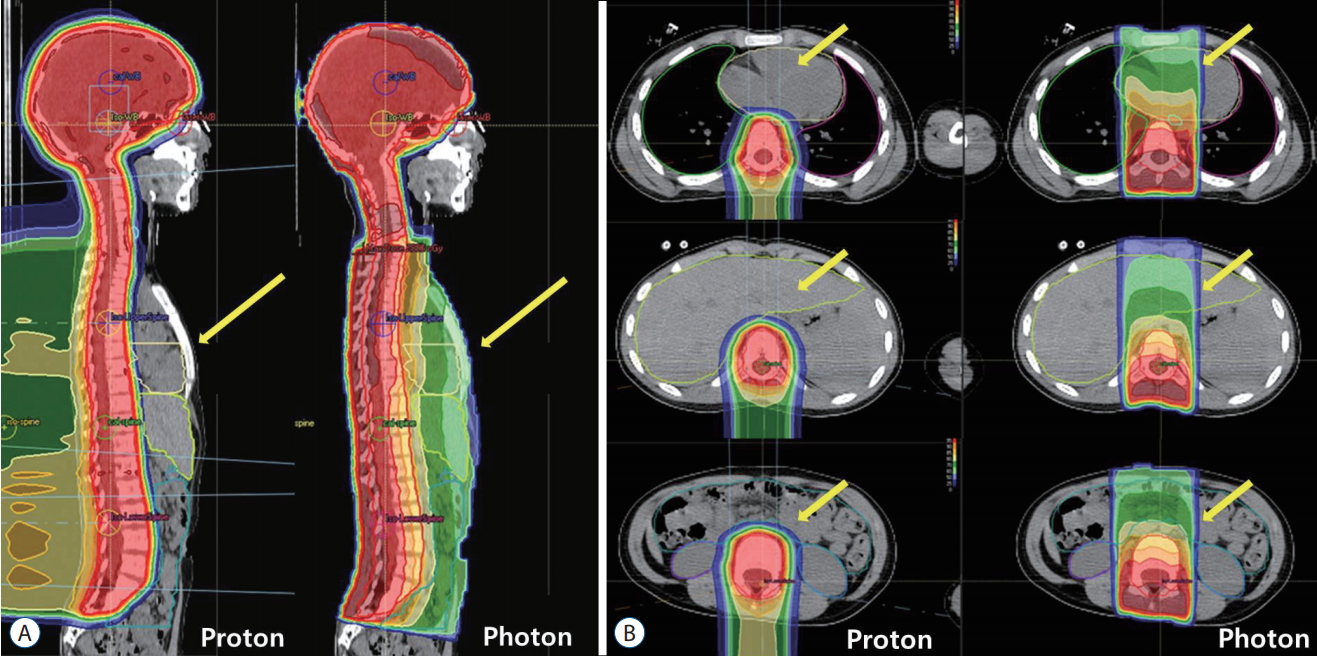
Sagittal (A) and axial (B) computed tomography images showing radiation dose distributions. Red lines show the planning target volume and red color areas are regions irradiated with 100% of prescribed doses. Proton beam has no exit dose beyond Bragg peak and yellow arrows show that there is little radiation to normal organs such as heart, liver and bowels which are located at the front of the radiation target (red lines) in proton beam therapy. It can decrease the risk of second malignancies from radiation.
Several studies reported the neurocognitive effect of proton beam therapy and compared with historical results of photon beam therapy. Generally those studies indicate that the use of proton beams can mitigate some of the neurocognitive sequelae from radiation therapy for CNS tumors. In one study, proton beam therapy rather than photon beam therapy reduced the volume of supratentorial brain and temporal lobe that received low or intermediate doses of radiation, and differences in the overall dose distributions showed that a reduction in radiation dose or volumes would have long-term, clinical advantages [12].
Another benefit of proton beam therapy over photon beam therapy is a reduction of risk for secondary malignancy and it might be the result of reduced radiation exposure of healthy tissue surrounding the tumor (Fig. 3). According to the Childhood Cancer Survivor Study [1], the cumulative incidence of all subsequent neoplasms in survivors of pediatric CNS tumors at 25 years after diagnosis is 10.7% although benign second neoplasms make up a significant proportion of these second tumors.
A matched cohort study [3] compared the risk of secondary malignancy in 558 patients treated with proton beam therapy at Harvard with that in another 558 patients’ data treated with photon beam therapy from The Surveillance, Epidemiology, and End Results. Proton beam therapy was associated with a lower incidence of second malignancies than photon beam therapy (6.9 vs. 10.3 per 1000 person-years, p=0.085). In a dosimetric study for medulloblastoma [31], researchers have calculated a risk of secondary malignancy after CSI with proton beam and photon beam. The predicted lifetime attributable risk was 4.6–10.0 fold higher with photon beam than with proton beam. These clinical and dosimetric studies strongly support the role of proton beam in diminishing the secondary malignancy risk, which is the most serious and life-threatening adverse effect of radiation for longterm survivors of pediatric malignant CNS tumors.
CONCLUSION
Survival in pediatric malignant CNS tumors continues to improve with the development of multidisciplinary approach and many efforts have been made to decrease treatment-related late toxicities. For radiation oncologists, these efforts included the developments of radiotherapeutic modality or techniques that can preserve more normal brain and the modification of radiation dose or irradiating volume according to tumor risk. In addition, the introduction of particle beams such as proton beam therapy for pediatric CNS tumors has a promise. The clinical application of proton beam therapy is increasing and the clinical results to date suggest that proton beam can reduce radiation-related late toxicities such as neurocognitive impairment and secondary malignancy. Therefore, proton beam therapy will become more widely available to children with malignant CNS tumors.
Notes
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.