Cold Allodynia after C2 Root Resection in Sprague-Dawley Rats
Article information
Abstract
Objective
The purpose of this study was to evaluate pain-related behaviors after bilateral C2 root resection and change in pain patterns in the suboccipital region in rats.
Methods
Male Sprague-Dawley rats were randomly assigned to three groups (n=25/group); näive, sham, and C2 resection. Three, 7, 10, and 14 days after surgery, cold allodynia was assessed using 20 μL of 99.7% acetone. c-Fos and c-Jun were immunohistochemically stained to evaluate activation of dorsal horn gray matter in C2 segments of the spinal cord 2 hours, 1 day, 7 days, and 14 days after surgery.
Results
Three days after surgery, the response to acetone in the sham group was significantly greater than in the näive group, and this significant difference between the näive and sham groups was maintained throughout the experimental period (p<0.05 at 3, 7, 10, and 14 days). Seven, 10, and 14 days after surgery, the C2 root resection group exhibited a significantly greater response to acetone than the näive group (p<0.05), and both the sham and C2 resection groups exhibited significantly greater responses to acetone compared with 3 days after surgery. No significant difference in cold allodynia was observed between the sham and C2 root resection groups throughout the experimental period. Two hours after surgery, both the sham and C2 root resection groups exhibited significant increases in c-Fos- and c-Jun-positive neurons compared with the naive group (p=0.0021 and p=0.0358 for the sham group, and p=0.0135 and p=0.014 for the C2 root resection group, respectively). One day after surgery, both the sham and C2 root resection groups exhibited significant decreases in c-Fos -positive neurons compared with two hours after surgery (p=0.0169 and p=0.0123, respectively), and these significant decreases in c-Fos immunoreactivity were maintained in both the sham and C2 root resection groups 7 and 14 days after surgery. The sham and C2 root resection groups presented a tendency toward a decrease in c-Jun-positive neurons 1, 7, and 14 days after surgery, but the decrease did not reach statistical significance.
Conclusion
We found no significant difference in cold allodynia and the early expression of c-Fos and c-Jun between the sham and C2 resection groups. Our results may support the routine resection of the C2 nerve root for posterior C1–2 fusion, but, further studies are needed.
INTRODUCTION
Posterior atlantoaxial segmental instrumented fusion can be technically challenging, but it is a highly successful surgical option for craniocervical junction pathology. Recently, polyaxial screw and rod fixation of the C1 lateral mas and C2 pedicle has gained popularity10,14,16,22). Goel et al.11–14) reported that in cases of C1–2 instability with basilar invagination, posterior atlantoaxial fusion is feasible through intraoperative cervical traction, followed by distraction of the C1–2 facet joint, and subsequently, firm lateral mass fixation11,12,14). They advocated that denuding the C1–2 articular cartilage and stuffing the bone graft within the C1–2 joint space can provide strong stability for C1–2 fixation. Based on their experience in treating more than 800 patients with atlantoaxial instability, most of them involving bilateral sectioning of the C2 root ganglion, they demonstrated that postoperative numbness in C2 dermatomes was well tolerated and did not lead to marked discomfort11–14). Although some studies have advocated C2 root sectioning for C1 lateral mass screw insertion13,15,21), C2 root resection for the placement of C1 lateral mass screws remains controversial.
Yeom et al.32) recommended against routine C2 nerve root transection when performing atlantoaxial segmental screw fixation. In their experience, more than a quarter of patients experienced an increase in neuralgic pain following C2 nerve root trasection. Therefore, remaining uncertain is whether C2 root resection is appropriate for posterior C1–2 fusion, likely because of a lack of studies that have specifically investigatd whether it affects patient outcome.
Randomized human studies of experimental applications of C2 root resection during posterior C1–2 fixation are very limited because of ethical considerations. Therefore, there is the need for a practical animal model of C2 root resection to gain insights into the pathomechanism and treatment of suboccipital neuralgia after C2 resection. To our knowledge, no study has evaluated suboccipital pain after C2 root resection in animals.
In the present study, we evaluated pain-related behavior after bilateral C2 root resection to investigate changes in pain patterns in suboccipital regions in rats. We analyzed the immunoreactivity of c-Fos and c-Jun, markers of neuronal activation and response to noxious stimulation, within 2 weeks after C2 root resection2,3,17,25,31).
MATERIALS AND MATHODS
All of the animal experiments were performed in accordance with the animal care guidelines of the National Institutes of Health, and were approved by the Institutional Animal Care Committee at Kyungpook National University Hospital (KNU 2016-99).
Seventy-five adult male Sprague-Dawley rats (11 weeks old) were purchased from Koatech Bio Inc. (Pyeongtaek, Korea) and acclimated to the laboratory environment for 1 week before the experiment. The rats were housed in an air-conditioned room (with 50% relative humidity) under a 12-hour/12 hours light/dark cycle at 23±2°C and given free access to food and tap water. The following week, the rats (at 12 weeks of age and weighing 340370 g) were randomly and blindly assigned to three groups (n=25 per group); naive, sham, and C2 resection. The rats were intraperitoneally anesthetized with a mixture of xylazine (10 mg/kg) and ketamine (90 mg/kg). The rats in the naive group were awakened after anesthesia and did not undergo surgery. The rats in the C2 root resection group underwent bilateral C2 root resection. Briefly, after the surgical site on the suboccipital and posterior neck region were shaved and prepared, the rats were placed prone on the operating table. A posterior midline skin incision was made over the upper cervical spine, and the posterior C1 arch and C2 lamina were exposed by dissecting the neck muscles. Careful dissection around the C1–2 joint was performed to find the C2 root ganglion just below the C1 posterior arch without any massive bleeding. Once exposed, the bilateral C2 roots were completely transected using microscissors at an area just immediately proximal to the dorsal root ganglion (Fig. 1). After surgery, the muscle, fascia, and skin were sutured using 4–0 prolene sutures, and postoperative antibiotics were given subcutaneously (0.5 mg/kg gentamicin). The rats in the sham group underwent an identical surgical procedure as the C2 root resection group but were not subjected to C2 root resection.
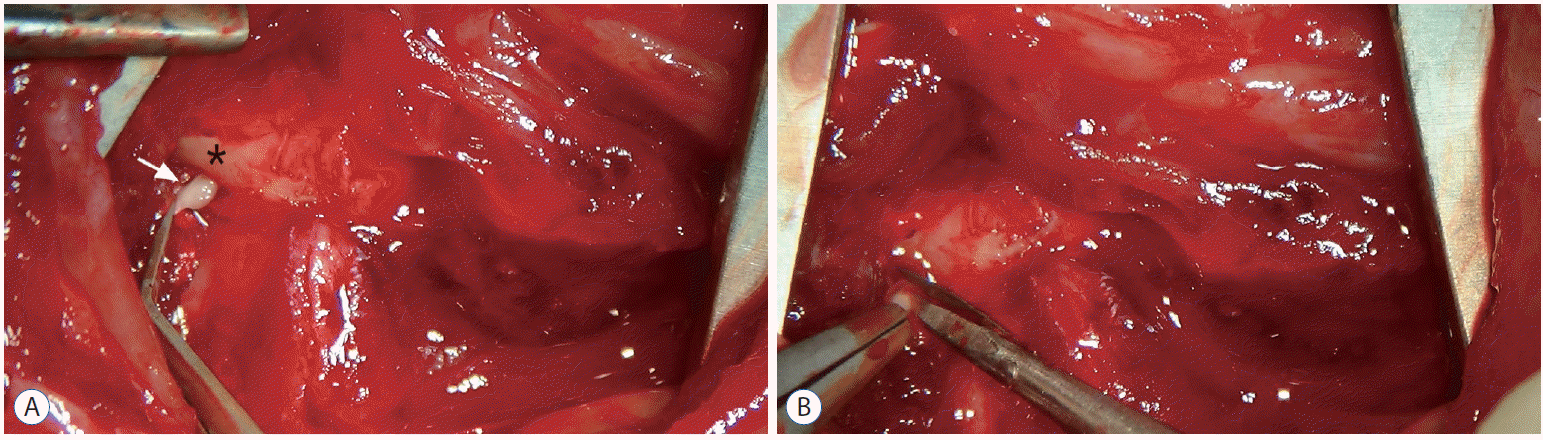
After careful dissection of the posterior neck muscle, the C2 root ganglion was revealed just below the C1 posterior arch. A : The white arrow indicates the C2 root ganglion. The asterisk indicates the posterior C1 arch. B : Once exposed, the bilateral C2 roots were completely transected using microscissor in an area just proximal to the dorsal root ganglion.
To avoid bias, the researcher, who conducted the behavioral test of cold allodynia, was blinded to the nature of the experimental manipulatios. Behavioral testing of cold allodynia was performed between 8:00 AM and 11:00 AM. On the experimental days, each rat was individually placed in a transparent plastic cage (25 cm width×18 cm height×35 cm length) in a quiet testing room for 30 minutes before behavioral measurements to allow the animal to adapt to the test environment.
For the behavioral test of cold allodynia, 20 μL of 99.7% acetone was dropped onto the glabrous surface below both ears (Fig. 2), and the number of episodes of forepaw scratching and aggressive behavior, including severe head shaking was recorded for 1 minute. We repeated the behavioral test three times at 5 minutes intervals, and the number of responses was averaged. Cold allodynia was examined at 3, 7, 10, and 14 days after surgery.
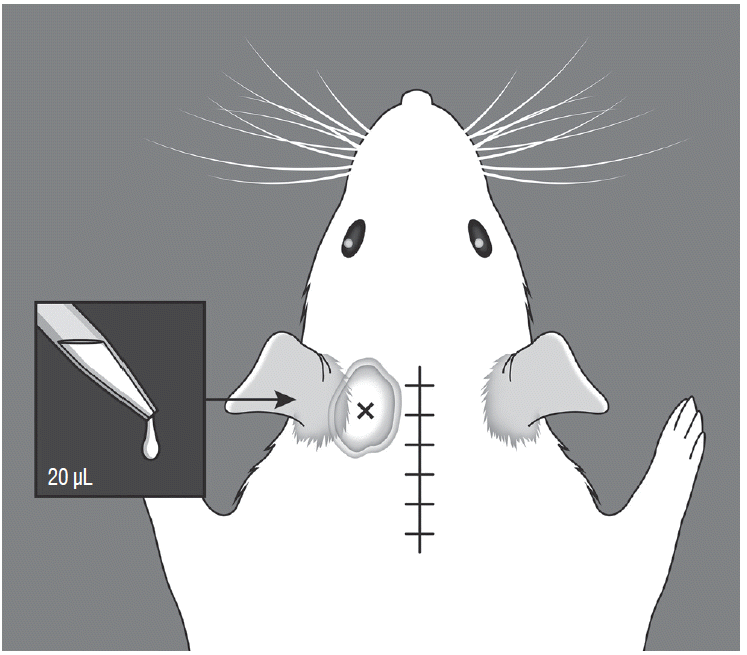
Illustration of cold allodynia testing. A volume of 20 μL of 99.7% acetone was dropped onto the glabrous surface below the ears. The acetone percolated through the suboccipital area, not cross the midline suture site.
For immunohistochemical staining, C2 segments of the spinal cord were extractred from the naive, sham, and C2 resection groups (n=46/group) 2 hours, 1 day, 7 days, and 14 days after surgery. The rats were then transcardially perfused with 150 mL of normal saline, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4) under deep anesthesia with a lethal dose of urethane (Sigma-Aldrich, St. Louis, MO, USA). Samples were postfixed in 4% paraformaldehyde overnight and then gradually dehydrated in ethanol and embedded in paraffin. Representative sections of the dorsal root entry zone were sliced into 3 μm-thick sections in the horizontal plane and mounted onto 0.02% poly-L-lysine-coated slides. Deparaffinized sections were quenched in hydrogen peroxide solution (10% H2O2 in PBS for 10 minutes), retrieved in citric acid (pH 6.0) for 20 minutes at microwave, incubated in 50 mM ammonium chloride (NH4Cl) for 30 minutes at room temperature, and blocked with blocking solution (1% bovine serum albumin, 0.2% gelatin, 0.05% saponin in PBS, and 5% normal goat serum) for 1 hour at room temperature. The sections were then incubated overnight at 4°C with c-Fos or c-Jun primary antibody (1 : 100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in PBS. The next day, after rinsing with PBS three times, the sections were incubated in biotinylated goat anti-rabbit secondary antibody (1 : 200 in PBS) and subsequently in an avidin-horseradish peroxide solution. Immunolabeling was visualized with 0.05 3, 3′-diaminobenzidine hydrochloride plus 0.3% hydrogen peroxide in PBS. The sections were then dehydrated with ethanol and xylene before mounting under coverslips with Permount.
Hematoxylin-eosin staining was used to determine morphological changes in the naive, sham, and C2 resection groups. Deparaffinized sections were dipped in Harris hematoxylin (Sigma-Aldrich) for 5 minutes, neutralized with 0.5% HCl and 0.05% ammonia water, dipped in eosin for 45 seconds, dehydrated in ethanol and mounted on the glasse slides. Photos of the stained sections were taken using a Zeiss Axioplan microscope (Carl Zeiss Meditec Inc., Jena, Germany). The images were viewed on a computer monitor using a Zeiss Plan-Apochromat 100× objective and Zeiss AxioCam HRc digital camera (Carl Zeiss Meditec Inc.). The analysis of c-Fos and c-Jun immunoreactivity was quantified by determining the number of c-Fos- and c-Jun- positive nuclei in dorsal horn gray matter of the spinal cord. The regions of gray matter corresponded to Rexed superficial laminae I–III and deeper laminae IV–VI (Fig. 3).
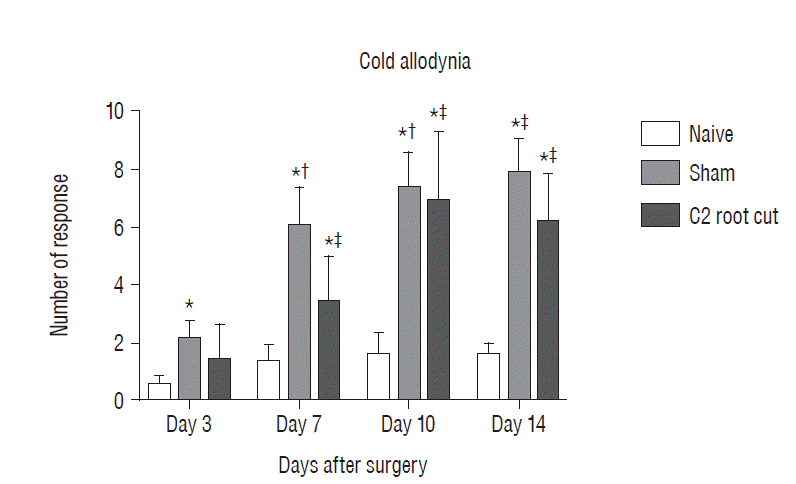
Evaluation pain-related behavior (cold allodynia) for 2 weeks after surgery. The data are expressed means±standard error of mean. *p <0.05, compared with naive group. †p <0.05, compared with sham group 3 days after surgery. ‡p<0.05, compared with C2 root resection group 3 days after surgery. No significant difference in cold allodynia was found between the sham and C2 root resection groups at each time point throughout the experimental period.
Statistical analysis
All of the statistical analyses were performed using Prism 7 software (GraphPad, La Jolla, CA, USA). The data are expressed as mean±standard error of mean. Repeated measure analysis of variance was for comparison between groups. Values of p<0.05 were considered statistically significant.
RESULTS
Postoperative pain-related behavior (i.e., cold allodynia) in response to 99.7% acetone showed difference among groups 3, 7, 10, and 14 days after surgery (Fig. 3). The naive group did not exhibit any significant differences in the response to acetone throughout the experiment period. The patterns of cold allodynia in both the sham and C2 resection groups were similar during the experiment. Three days after surgery, the response to acetone in the sham group was significantly greater than in the naive group, and this significant difference between the naive and sham groups was maintained throughout the experimental period (p<0.05 at 3, 7, 10, and 14 days). The C2 root resection group exhibited a greater response to acetone 3 days after surgery, but this response did not reach statistical significance compared with the naive group (p>0.05). Seven, 10, and 14 days after surgery, the C2 root resection group exhibited a significantly greater response to acetone compared with the naive group (p<0.05). Seven, 10, and 14 days after surgery, both the sham and C2 resection groups exhibited significantly greater responses to acetone compared 3 days after surgery. However, no significant difference in cold allodynia was observed between the sham and C2 root resection groups at each time point throughout the experimental period.
Histologic examination of C2 segments of spinal cords did not show any morphological changes in all three groups throughout the experimental periods (Fig. 4). Immunohistochemical staining of c-Fos and c-Jun in C2 segments of the spinal cord showed that, in the naive group, there were no significant changes in c-Fos and c-Jun immunoreactivity throughout the experimental period (p>0.05, Figs. 5 and 6). Two hours after surgery, both the sham and C2 root resection groups exhibited significant increases in c-Fos- and c-Jun-positive neurons compared with the naive group (p=0.0021 and p=0.0358 in the sham group, and p=0.0135 and p=0.014 in the C2 root resection group, respectively). One day after surgery, both the sham and C2 root resection groups exhibited significant decreases in c-Fos-positive neurons compared with 2 hours after surgery (p=0.0169 and p=0.0123, respectively), and these significant decreases were maintained in both the sham and C2 root resection groups 7 and 14 days after surgery.
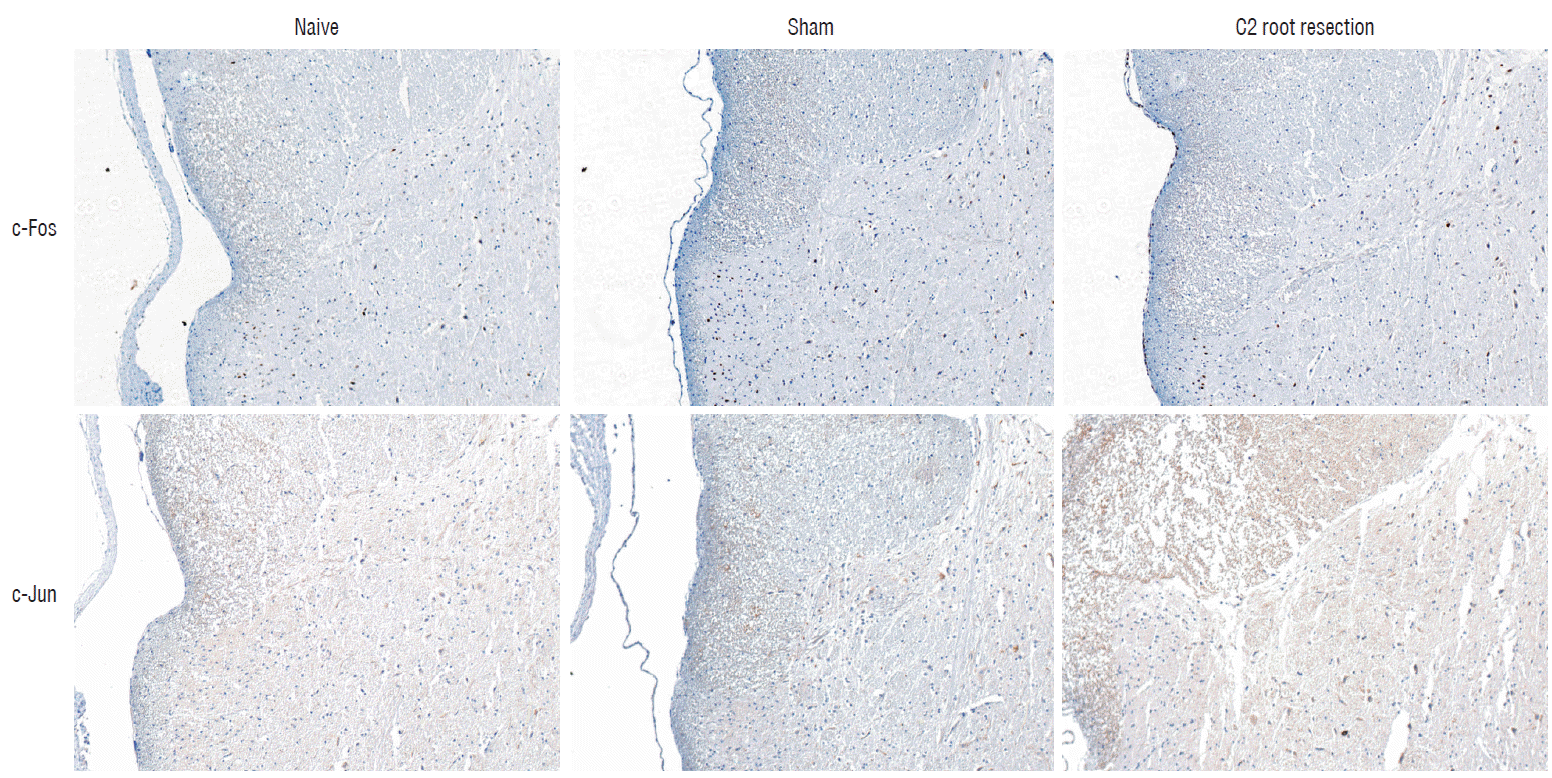
Immunohistochemical staining of c-Fos and c-Jun. Representative photographs of c-Fos- and c-Jun-postive immunoreactivity cells from dorsal horn gray matter of C2 segments of spinal cord at 1 day after experiment. 100×magnification.

Immunohistochemical staining of c-Fos (A) and c-Jun (B) in C2 segments of the spinal cord. *p <0.05, compared with naive group. †p <0.05, compared with sham group 2 hours after surgery. ‡p <0.05, compared with C2 root resection group 2 hours after surgery.
Both the sham and C2 root resection groups presented a tendency toward a decrease in c-Jun-positive neurons 1, 7, and 14 days after surgery, but this decrease did not reach statistical significance.
DISCUSSION
Since the first introduction by Harms and Melcher polyaxial screw and rod fixation of the C1 lateral mass and C2 pedicle has gained popularity for craniovertebral junction pathology10,14,16,22). Originally, Harms and Melcher16) did not sacrifice the C2 nerve root and instead used a C2 screw with a smooth unthreaded portion to minimize irritation of the C2 nerve root. They achieved fusion in 100% of their series of 37 patients and did not encounter any neural complications including C2 neuralgia. However, recent studies have advocated routine transection of the C2 root for posterior C1–2 fusion, because it can improve exposure of the screw insertion sites and C1–C2 facet joints, increase the fusion bed area, and allow the easier control of bleeding from the venous plexus at C1–C29,11,12,15). Goel et al.11–14) demonstrated that postoperative numbness in C2 dermatomes after bilateral section of the C2 ganglion is well tolerated and does not lead to any marked discomfort. The numbness and heaviness on the back of the head progressively lessens in intensity and extent with time11–14). In a recent study by Hamilton et al.15), 80% of patients in their series had preoperative occipital neuralgia. In all of these patients, this neuralgia was relieved by C1–2 instrumented arthrodesis with C2 neurectomy.
However, remaining controversial is whether preservation or transection of the C2 nerve root results in a reduction of postoperative occipital neuralgia. A recent study by Yeom et al.32) showed that more than a quarter of the patients in their series experienced an increase in neuralgic pain following C2 nerve root transection. Therefore, these authors argued against routine C2 nerve root transection when performing atlantoaxial segmental screw fixation32). These opposing viewpoints regarding suboccipital neuralgia after C2 nerve root transection raised the need to perform further well-designed prospective randomized controlled studies.
Animal models provide a systematic and well-controlled experimental environment, and are useful for identifying potential therapeutic targets for neuropathic pain28). In the present study, we used Sprague-Dawley rats to investigate behavioral responses to pain in the suboccipital area after bilateral C2 root resection.
Allodynia is defined as a painful response to normally innocuous stimuli. Hyperalgesia is defined as increased in the pain response to normally painful stimuli. Both allodynia and hyperalgesia are major components of neuropathic pain, which refers to pain that originates from pathology of the nervous system, including the central nervous system (e.g., spinal cord) and peripheral nerves. In neuropathic pain, tissue damage directly affects the nervous system, resulting in the generation of ectopic discharges that bypass transduction4,6,24). Persistent postoperative pain can be a consequence of nerve injury during surgery, and abnormal signals arise from both injured axons and intact nociceptors that share the innervation territory of the injured nerve4,28).
In the present study, we evaluated cold allodynia in the suboccipital area in Sprague-Dawley rats after bilateral C2 root resection. Both the sham and C2 resection groups presented similar patterns of cold allodynia during the experiment. Three days after surgery, the response to acetone in the sham group was greater than in the naive group, and this difference was maintained throughout the experimental period. The C2 root resection group did not exhibit a significant difference in cold allodynia compared with the sham group at any of the time points after surgery. Although cold allodynia in an animal model dose not soley reflect the overall characteristics of neuropathic pain, our results support routine transection of the C2 nerve root for posterior C1–2 fusion, as suggested by Goel et al.11–14). Among the various animal models of neuropathic pain7,18,23,24), the spared nerve injury model was first developed by Decosterd and Woolf in 2000 in Sprague-Dawley rats to enhance the reproducibility of injury and behavioral responses to partial nerve injury7). In rats, cold allodynia developed immediately after spared nerve injury, and was maintained for several months during the observation period, as well as other components of neuropathic pain such as heat hyperalgesia, mechanical allodynia and mechanical hyperalgesia7). With regard to cold allodynia, the present results were consistent with this previous study.
The present study used two markers of neuronal activation; c-Fos (the protein product of the immediate-early gene, c-fos), and c-Jun (the protein product of the immediate-early gene, c-jun). These two markers have been widely used to study the neural correlates of nociception, neuronal activation and noxious stimulation2,3,17,25,31). A recent study by Li et al.25) reported that the number of c-Fos- and c-Jun-positive neurons in ipsilateral C5–7 segments significantly increased 2 and 4 hours after C7 nerve root rhizotomy compared with the sham group. The localization of c-Fos- and c-Jun-positive neurons in C5–7 gray matter was mainly found in lamina IX of the anterior horn and laminae I–II of the dorsal horn of the spinal cord. The number of c-Fos- and c-Jun-positive neurons in C5–7 gray matter significantly decreased 4 hours after surgery compared with 2 hours after surgery. Our results also showed a similar pattern of c-Fos and c-Jun expression after C2 root resection. In the present study, the C2 root resection group exhibited a significant increase in c-Fos and c-Jun expression 2 hours after surgery compared with the naive group, and a significant decrease in c-Fos expression 1 day after surgery compared with 2 hours after surgery. These findings are consistent with the previous study. In the present study, the sham group also exhibited a significant increase in c-Fos- and c-Jun-positive neurons, which is opposite to the findings of Li et al.25). These diametrically opposing results in the sham group reflect the different animal species or different models used.
In humans, the anatomy of the C2 nerve root and its ganglion has been studied in detail. The C2 ganglion lies in the intervertebral space between C1 and C2, bordered by the posterior arch of the atlas, lamina of the axis, and atlantoaxial joint. The dorsal ramus of the C2 spinal nerve emerges between the posterior arch of the atlas and lamina of the axis, and the medial branches of the ramus terminate as the greater occipital nerve. After piercing the trapezius aponeurosis, the greater occipital nerve travels with the scalp as far anterior as the vertex of the skull8,26,29,30). However, no data are available regarding the anatomy of the C2 nerve root and its innervated dermatomes in the suboccipital area in animals. We assumed that rats also have similar passages and innervations of the C2 nerve root. Therefore, we applied acetone to the glabrous surface below both ears. Acetone (20 μL) percolated through the suboccipital area, but did not cross the midline suture site. We supposed that if the acetone crossed the midline suture site, then this would cause additional nociceptive incisional pain instead of neuropathic pain. Our preliminary study confirmed that 20 μL acetone was sufficient to percolate through the suboccipital area in rats, and not cross the midline suture site (data not shown).
To test pain-related behavior in animals, most studies manipulate the plantar surface of the hindpaw, where there is no fur1,3,5,19,20,27,28). In the present study, although the surgical site in the suboccipital and posterior neck region was thoroughly shaved after anesthesia, minute amount of fur grew several days after shaving, which may hinder the precise testing of cold allodynia. We did not shave the region again, because, we thought that re-shaving with repeated anesthesia would be stressful to the animals, and thus affect pain-related behavior.
In the present study, we did not evaluate mechanical allodynia using a series of von Frey filaments, which is most commonly used to test pain-related behavior in animal models of neuropathic pain1,20). To our knowledge, no reference data are available regarding mechanical allodynia after C2 root resection in animals. Mechanical allodynia testing in rat would be difficult to condut in the suboccipital region rather than the hindpaw.
CONCLUSION
The present study found no significant difference in cold allodynia and early expression of c-Fos and c-Jun between the sham and C2 resection groups. Our results may support the routine resection of the C2 nerve root for posterior C1–2 fusion, but further studies are needed.
Notes
CONFLICTS OF INTEREST
The authors have no financial conflicts of interest.
INFORMED CONSENT
This type of study does not require informed consent.