Complications Following Transradial Cerebral Angiography : An Ultrasound Follow-Up Study
Article information
Abstract
Objective
The feasibility and usefulness of transradial catheterization for coronary and neuro-intervention are well known. However, the anatomical change in the catheterized radial artery (RA) is not well understood. Herein, we present the results of ultrasonographic observation of the RA after routine transradial cerebral angiography (TRCA).
Methods
Patients who underwent routine TRCA with pre- and post-procedure Doppler ultrasonography (DUS) of the catheterized RA were enrolled. We then recorded and retrospectively reviewed the diameter and any complicated features of the RA observed on DUS, and the factors associated with the diameter and complications were analyzed.
Results
A total of 223 TRCAs across 181 patients were enrolled in the current study. The mean RA diameter was 2.48 mm and was positively correlated with male gender (p<0.001) and hypertension (p<0.002). The median change in diameter after TRCA was less than 0.1 mm (range, -1.3 to 1.2 mm) and 90% of changes were between -0.8 and +0.7 mm. Across 228 procedures, there were 12 cases (5.3%) of intimal hyperplasia and 22 cases (9.6%) of asymptomatic local vascular complications found on DUS. Patients with abnormal findings on the first procedure had a smaller pre-procedural RA diameter than that of patients without findings (2.26 vs. 2.53 mm, p=0.0028). There was no significant difference in the incidence of abnormal findings for the first versus subsequent procedures (p=0.68).
Conclusion
DUS identified the pre- and post-procedural diameter and local complications of RA. Routine TRCA seems to be acceptable with regard to identifying local complications and changes in RA diameter.
INTRODUCTION
Transradial coronary intervention has become the preferred mode of coronary intervention given its simplicity and the easy hemostasis of the superficially located radial artery (RA). Due to abundant collateral circulation in the palm from the ulnar side, ischemic complications seldom occur even after radial artery occlusion (RAO)2,15,26). There are multiple reports on the safety of transradial angiography in terms of radiation dose, procedure quality, survival, and local complications in the coronary literature15,27,30,31).
In neuroangiography, given the familiarity, easy accessibility, and low complication rate of transfemoral procedures, almost all procedures are performed via the femoral route. There are only a few published reports on transradial cerebral angiography (TRCA) in the literature, and they focus primarily on its feasibility and technical aspects10,12,16,18,19,24,33,35). Since 2007, we have routinely performed TRCA at our center due to the advantages of patient comfort and convenient hemostasis. Since January 2011, we have prospectively collected the data of patients who underwent elective TRCA, especially data regarding morphological changes of the RA on Doppler ultrasonography (DUS).
In this study, we demonstrate the DUS findings of the catheterized RA, including the diameter and complicating features, and discuss the safety of TRCA. To our knowledge, this is the first study that addresses the observation of RA changes using DUS in the field of neuro-endovascular surgery.
MATERIALS AND METHODS
We employed the radial artery as the primary site of access for performing cerebral angiography. Transfemoral access or another alternative was used in the following situations: weak RA pulsation, abnormal Allen’s test, abnormal Barbeau’s test, RA diameter below 1.5 mm on preoperative DUS, candidacy for kidney dialysis or coronary bypass surgery, RA access failure, anatomical variation that hindered catheter access over the supra-aortic vasculature, and refusal of the transradial approach.
All of the patients were premedicated with 100 mg aspirin orally for at least 7 days prior to the procedure. If the intervention was anticipated soon after the angiography, 75 mg Plavix (clopidogrel; Sanofi-aventis, Paris, France) was prescribed in addition to aspirin.
Demographic features including age, gender, and risk factors (hypertension, diabetes, hypercholesterolemia, and smoking) were recorded.
Pre-procedural evaluation of palmar collateral circulation and Doppler ultrasonography
Pre-procedural tests for palmar collateral circulation and DUS were performed on all patients. Recovery of capillary blushing within 9 seconds after releasing ulnar artery compression was accepted as a normal modified Allen’s test3). Recovery of oxygen pulse tracing on plethysmography/oximetry within 2 minutes after releasing ulnar artery compression was recorded as a normal Barbeau’s test. Patients with abnormal collateral circulation test were transitioned to transfemoral cerebral angiography. The pre-procedural DUS (S-cath; SonoSite, Inc., Bothel, WA, USA) was performed by a sonographer on patients who passed the collateral circulation tests2,5,31). Both the radial and ulnar arteries were checked for diameter, patency, and tortuosity along their course. The diameter was measured from intima to intima on the anticipated puncture site4).
Transradial catheterization procedure
The right forearm including the hand, as well as bilateral femoral sites, were prepared aseptically. The right arm was placed on an accessory oblique board in a position that was 30 degrees abducted, supinated, and slightly extended using a wrist pillow and a pulse oximeter placed on the right thumb. A 5-French (Fr) 7 cm sheath with a dilator (Terumo, Somerset, NJ, USA) was then inserted into the RA using a standard Seldinger technique. We administered 3000 U of heparin intravenously and a 7-mL radial cocktail solution consisting of 5 mg of verapamil, 200 mg of nitroglycerin, and 1000 IU of heparin was administered intra-arterially through the sheath. The routine TRCA was completed using 5 Fr Simmons 2 catheter (Terumo).
Hemostasis and postoperative care
Hemostasis was achieved using the following protocol : TR band (Terumo) application on the puncture site, withdrawal of the sheath by 5 cm, gradual removal of the sheath with simultaneous balloon infusion to stop bleeding, slow deflation to check for bleeding recurrence, and then slight re-inflation. We evaluated the radial artery proximal and distal to the TR band using DUS for a to-and-fro Doppler pattern implying patency of the radial artery. The band was kept for 2 hours and then gradually deflated for another hour. A simple bandage was applied on the puncture site after removal of the TR band. Before discharge, RAO or other local complications were checked by palpation of the RA and the reverse Allen’s test. All patients were prescribed 7 days of oral aspirin.
Follow-up Doppler ultrasonography
A 7-day follow-up DUS was performed by an independent radiologist. Radial and ulnar arteries were examined up to the antecubital area while the diameter, Doppler flow and wave, morphological changes, and complications were noted. The primary endpoint of this study was to identify local vascular complications such as RAO and other miscellaneous complications that were observed by DUS. The secondary endpoint was changes in the RA diameter after TRCA.
Data analysis
We used univariate and multivariate mixed-effects regression to test the effect of various factors on the pre-procedural artery diameter and the post-procedural changes in artery diameter. Differences in the proportions between groups were tested by Fisher’s exact test. Differences in continuous variables between groups were tested by the Kruskal-Wallis and Wilcoxon tests. All statistical analyses were performed with Stata release 13.1 (StataCorp LP, College Station, TX, USA). A p-value <0.05 was considered significant.
RESULTS
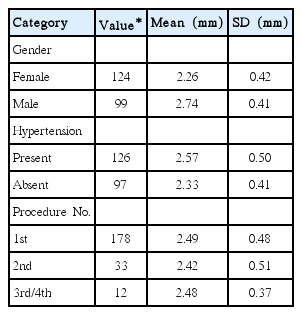
From January 2011 to November 2013, a total of 223 TRCA procedures were performed in 181 patients and pre-procedure DUS was carried out. Follow-up DUS was obtained on the seventh day after the procedure and these patients were enrolled (Table 1). The median time between procedures for those patients who underwent more than one procedure was 7 months (interquartile range, 5–10).
Radial artery diameter
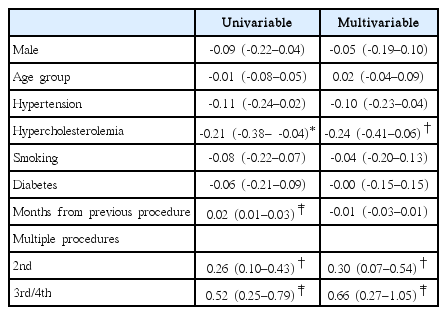
The mean pre-procedure RA diameter was 2.48 mm (standard deviation, 0.47; range, 1.5–3.4 mm). Larger pre-procedural diameter was significantly associated with male gender (p<0.001) and the presence of hypertension (p<0.002) after controlling for age, smoking, number of procedures, and hypercholesterolemia (Table 2). The median change in the RA diameter after the first procedure was less than 0.1 mm (range, -1.3 to 1.2 mm) and 90% of the changes in diameter were between -0.8 and +0.7 mm. Patients with hypercholesterolemia had significant reductions in artery diameter compared to patients without hypercholesterolemia (p<0.002) after the first procedure. Results of a mixed-effects regression model, which treated subjects as random effects, are presented in Table 3. After controlling for demographic, behavioral, and comorbid factors, changes in RA diameter were significantly associated with presence of hypercholesterolemia (p<0.01). RA diameter changes were also significantly associated with multiple procedures versus the initial procedure (second [p<0.01] and third/fourth [p<0.001]).
Abnormal findings on follow-up DUS
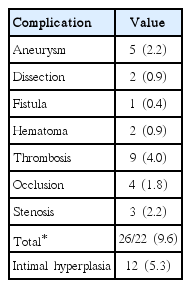
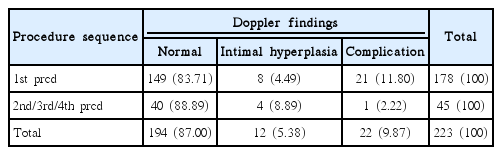
Across 223 procedures, we detected 12 cases (5.4%) of intimal hyperplasia without significant blood flow restriction and 22 cases (9.9%) of local vascular complications (Table 4). The complications were all asymptomatic. Some of the cases with complications included multiple features simultaneously, such as thrombus with aneurysm and stenosis with intraluminal thrombus. There was no significant difference in the incidence of abnormal findings for the first versus the subsequent procedures (p=0.68). Patients undergoing their third or fourth procedure demonstrated no complications, and 4 of the 12 intimal hyperplasia cases occurred on the second or later procedures (Table 5). Patients with abnormal findings on the first procedure had smaller pre-procedural RA diameters than those of patients without findings (2.26 vs. 2.53 mm, p=0.0028) (Table 6). The average change in diameter on the first procedure was the same for the patients with and without abnormal findings (p=0.76). However, patients with abnormal findings showed greater variability in the RA diameter change (p=0.011, by robust test of variance). And there were no cases of intracranial hemorrhagic or ischemic complications following the TRCA.
DISCUSSION
RA as an alternative access route
Due to its superficial location, the RA can be easily accessed if the pulse is palpable and bleeding can be easily controlled. In addition, a hematoma can be detected quickly due to the lack of excess soft tissue in the wrist. Its relatively straight course to the aortic arch simplifies the navigation of the catheter and wire. Given the abundant collateral circulation from the ulnar artery that forms the deep and superficial palmar arches, ischemic symptoms seldom occur even if the RA occludes after catheterization. Patients do not need to lay flat and immobilized for hemostasis, contrary to the transfemoral artery approach (TFA). These features encourage physicians to use the RA as an alternative route for coronary catheterization6,13,15,25,31). Many clinical trials have supported the transradial approach (TRA) over the TFA for coronary intervention. In the Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome study, Romagnoli et al.25) concluded that the TRA is associated with less morbidity and mortality compared to the TFA. The randomized, parallel, and multicenter RIVAL study found that both approaches were effective and safe for percutaneous coronary intervention (PCI) in acute coronary syndrome, but that the TRA was significantly associated with fewer local complications13). There are several reports regarding the TRA in the neurointervention arena. However, they are limited to series demonstrating the feasibility and experience of the use of this technique10,12,16,18,19,24,33,35). Jo et al.12) demonstrated the feasibility of TRCA by analyzing the success rate in the largest series of 1240 cases. Our current study deals with routine TRCA cases evaluated by DUS for investigation of actual arterial change in terms of local complications and RA diameter.
Radial artery diameter, multiple procedures, and intimal hyperplasia
Our results revealed a significant relationship between hypercholesterolemia and the RA diameter change. In the presence of hypercholesterolemia, the post-procedural RA diameter change showed a statistically significant difference. The effect of high cholesterol level on either arterial diameter or microcirculation is not yet clearly understood. However, there are some recent researches insisting on the negative impact of high cholesterol on endothelial impairment as well as worsened microcirculation17), and there is no doubt that these changes lead to the dysfunction and compromise of vascular structures8). These structural changes include the narrowing of the inner diameter of vessels, and our results are compatible with this general consensus. Data analysis showed a positive relationship between a larger RA diameter, male gender, and hypertension7). Compared with female patients, male patients usually have larger wrists and greater muscle mass, and those anatomical differences might explain this tendency14).
A smaller radial artery diameter was associated with local complications. In terms of the technical success of cannulation, easier cannulation was associated with a larger RA diameter. Smaller RAs with weaker pulsation can necessitate more cannulation attempts, which may lead to complications or intimal injury9,32).
Multiple procedures on the same RA did not pose an additional risk for local complications. However, the diameter change seen with multiple procedures was greater than the change in RA diameters after one procedure. Wakeyama et al.32) observed intimal-medial thickening after catheterization, which led them to believe that catheterization could damage the intimal cell layer and decrease medial elasticity. The possibility that RA catheterization may influence arterial wall function can be explained by the incidence of intimal hyperplasia, which was 5.3% in the current study. Long-term structural adaptation with significant intimal-medial thickening was also observed by the Stegemann group27). Yan et al.34) demonstrated a decrease in the flow-mediated-dilatation and nitroglycerine-mediated-dilatation of RAs measured by ultrasound following transradial coronary procedures. Hence, multiple procedures on the same RA could lead to a higher possibility of wall remodeling and functional deterioration. In our series, 4 of 12 cases with intimal hyperplasia were observed in the multiple procedure group, which was twice as many as in the single procedure group (Table 5). Our results are compatible with those of previous studies conducted on transradial coronary angiographies and they demonstrate the value of DUS as a prediction tool for complications following the procedure. Our results have novel value, as there is a scarcity of studies regarding the use of DUS for transradial ‘cerebral angiographies’.
Local complications
In this study, local complications were categorized as hemorrhagic, occlusive, or miscellaneous. Hemorrhagic complications included a large hematoma that required evacuation and a small hematoma that could be observed. TRA is associated with significantly fewer hemorrhagic complications than the femoral approach25). The current study showed no major hemorrhage and 2 cases (0.9%) of small and asymptomatic hematomas detected only on DUS.
Another major known complication is RAO. Even patients with normal palmar collateral circulation tests before the transradial procedure can encounter severe ischemic symptoms that warrant treatment. Many physicians apply temporary occlusion of the ulnar artery and anticoagulation in the form of low molecular weight heparin or intravenous heparin injections for recanalization1,11,20,21,36). While operative treatment may be required for some patients, RAO is usually asymptomatic. RAO can preclude radial artery use for access and as a donor for future bypass surgery. The rate of RAO varies from 5% to 30% depending on the tool used for diagnosis, with ultrasound being the most accurate4,11,21,23,26,28-30,36). In a large prospective study, Uhlemann et al.30) described the advantage of ultrasonography for the investigation of local complications in patients who underwent transradial PCI. They described a high rate of RAO of 13.7% for the 4 Fr sheath group and 30.5% for the 6 Fr sheath group30). Zankl et al.36) reported that 10.5% of patients had a partial or complete RAO on duplex ultrasonography immediately after transradial coronary intervention. In the current study, there were 3 cases of RAO (1.3%), 9 cases of thrombus (9.0%), and 5 cases of stenosis (2.2%). Radial artery occlusion was found to be secondary to intraluminal occlusive phenomena rather than external hematoma compression. On ultrasonography, iso- to hyperechoic thrombi were detected in the lumen of occluded radial arteries, while progressive intimal hyperplasia was observed in one patient with obstructive Doppler patterns (Fig. 1). We could not conclude whether this was an immediate post-procedural occlusion or a delayed phenomenon. No treatment was applied to these cases of RAO and thrombosis due to the absence of symptomatic lesions. Periprocedural antithrombotic agents and patent hemostasis seem to be crucial to achieve a low rate of complete RAO20,22,23). Our protocol of follow-up DUS at 1 week after the procedure might have influenced these favorable results by allowing time for spontaneous healing. Previous studies on this subject demonstrated a method in which DUS was performed within 24 hours after the procedure.
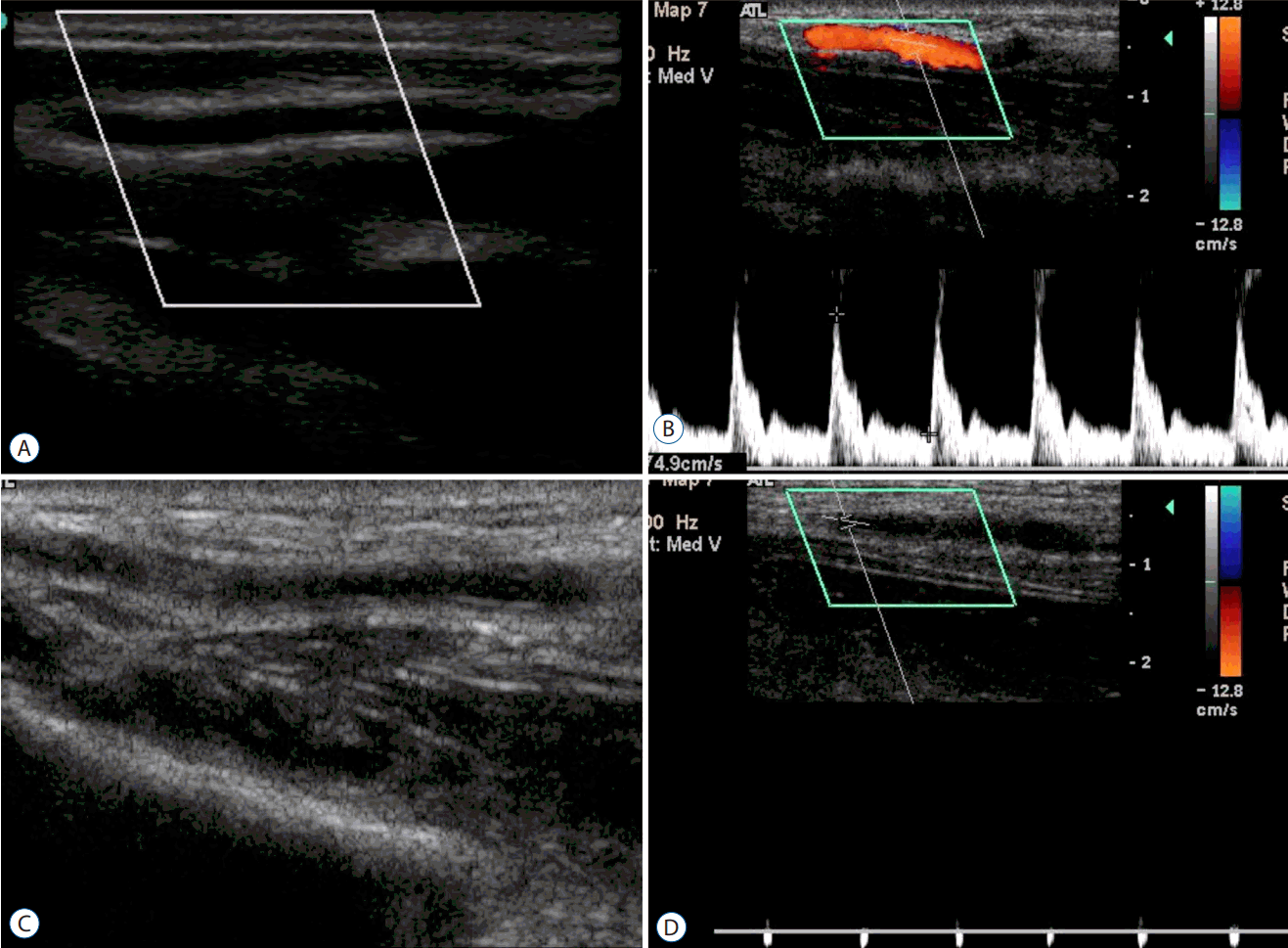
A case of radial artery occlusion. A : This shows a pre-procedural normal radial artery with an intact lumen and vessel wall. B : This is the normal Doppler wave-form of the ulnar artery at the post-procedure follow-up. C : In contrast to (A) and (B), there is vague echogenicity in the lumen, which is assumed to indicate thrombus formation. D : The Doppler shows an obstructive wave pattern.
The observed miscellaneous complications included aneurysms and arteriovenous fistulas, all of which were asymptomatic (Fig. 2). It was interesting that multiple procedures did not increase the risk of these complications. The median time between procedures for those patients who underwent more than one procedure was 7 months, which may have allowed time for intimal healing and regeneration.
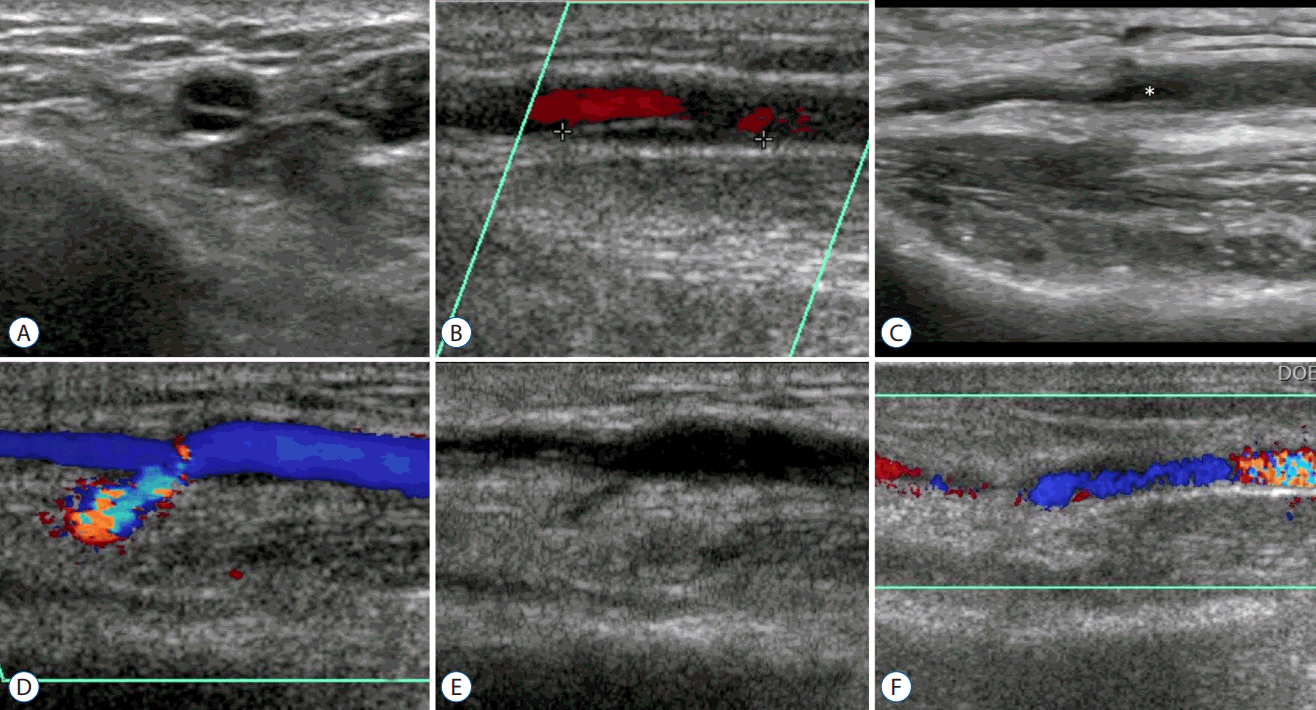
Doppler ultrasonographic findings of various complications. A and B : A dissection of a radial artery in cross-sectional and longitudinal view. Note the double lumen sign. In the Doppler image that is not shown in this figure, the false lumen did not have any blood flow signal. C : An aneurysmal change in the radial artery around the puncture site (asterisk). D and E : A pseudoaneurysm case in Doppler and gray scale images. The pseudoaneurysm sac does not appear to have a proper wall and shows mixed Doppler signals due to the turbulent blood flow. F : A case of arteriovenous fistula.
Limitations
This study still has some limitations. Due to the retrospective observational nature of this analysis, only a limited number of factors were included in this study, and there might be a possibility of selection bias. Moreover, a comparison group is lacking, such as a transfemoral approach group, due to the nature of this study. Another major limitation is that this study does not include the functional outcome following the DUS imaging of the RA. Although we assessed the complication rate, we did not evaluate the functional changes under physiologic range, such as muscle strength or subjective discomfort of the procedure site after TRCA. These factors should be investigated in further studies.
Another limitation of the current study is, that due to the insurance problem and lack of financial support, we could not perform additional follow-up study for the patients with abnormal findings in early 7th day DUS. Long term follow-up studies, and a comparative analysis with transfemoral approach as well as functional outcome studies are needed.
CONCLUSION
When performing a TRCA, DUS enables clinicians to track the pre- and post-procedural RA diameter and identify local complications. Transradial catheterization did not significantly alter the RA diameter and a smaller diameter was associated with the risk of local vascular complications. The rate of complications assessed by follow-up DUS was acceptable and a significant proportion of steno-occlusive features were observed.
Acknowledgements
We would especially like to thank Jarret Rosenberg, who did the initial statistical analysis of this study. This work was approved by the Catholic University of Korea Institutional Review Board (IRB No. PC14RISE0049).