Brain Abscesses Associated with Asymptomatic Pulmonary Arteriovenous Fistulas
Article information
Abstract
Brain abscess commonly occurs secondary to an adjacent infection (mostly in the middle ear or paranasal sinuses) or due to hematogenous spread from a distant infection or trauma. Pulmonary arteriovenous fistulas (AVFs) are abnormal direct communications between the pulmonary artery and vein. We present two cases of brain abscess associated with asymptomatic pulmonary AVF. A 65-year-old woman was admitted with a headache and cognitive impairment that aggravated 10 days prior. An magnetic resonance (MR) imaging revealed a brain abscess with severe edema in the right frontal lobe. We performed a craniotomy and abscess removal. Bacteriological culture proved negative. Her chest computed tomography (CT) showed multiple AVFs. Therapeutic embolization of multiple pulmonary AVFs was performed and antibiotics were administered for 8 weeks. A 45-year-old woman presented with a 7-day history of progressive left hemiparesis. She had no remarkable past medical history or family history. On admission, blood examination showed a white blood cell count of 6290 cells/uL and a high sensitive C-reactive protein of 2.62 mg/L. CT and MR imaging with MR spectroscopy revealed an enhancing lesion involving the right motor and sensory cortex with marked perilesional edema that suggested a brain abscess. A chest CT revealed a pulmonary AVF in the right upper lung. The pulmonary AVF was obliterated with embolization. There needs to consider pulmonary AVF as an etiology of cerebral abscess when routine investigations fail to detect a source.
INTRODUCTION
The cerebral abscess is a common central nervous system infection that can result from trauma, hematogenous spread, or spread from an adjacent infection such as otitis media or sinusitis. Despite exhaustive searches, 15 to 30% of abscesses are termed cryptogenic when no source of infection is identified20). A distant infection focus that can cause brain abscesses is a cardiac right to left shunt, which is related to patent foramen ovale15), cyanotic cardiac disease15) or pulmonary arteriovenous malformation or fistula (AVF). Pulmonary AVF is a rare congenital vascular malformation involving direct communication between the pulmonary artery and vein without an intervening capillary bed. Approximately 80–95% of pulmonary AVFs are associated with hereditary hemorrhagic telangiectasia (HHT), known as Osler-Weber-Rendu syndrome5,8,9). The clinical triads of pulmonary AVF is cyanosis, exertional dyspnea, and digital clubbing; however, 56% of one large series were asymptomatic8). The most prominent central nervous system (CNS) complications associated with pulmonary AVFs are neurologic events, including transient ischemic attacks31), recurrent stroke1,6,18,27), brain abscesses, and seizures26). Reports of asymptomatic idiopathic pulmonary AVF-related brain abscesses are rare. We will focus on the cryptogenic brain abscess as developed by patients with idiopathic pulmonary AVF, which may be detected only if we consider it may cause the brain abscess.
CASE REPORT
Case 1
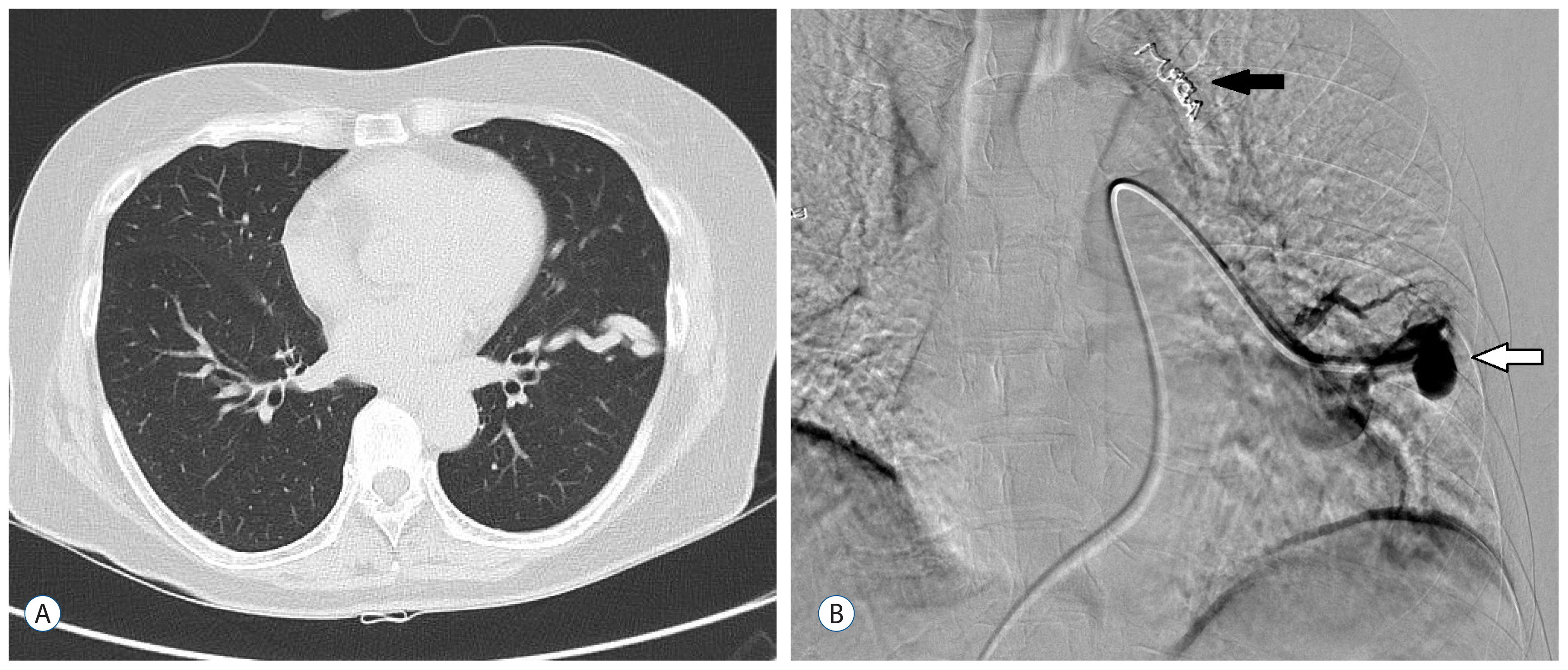
A 65-year-old woman was admitted with a 1-month history of headache and cognitive impairment that had become aggravated 10 days prior. She showed impairment in orientation and judgment, but there were no lateralization signs of motor paralysis or cranial nerve deficits. Her past medical history was unremarkable except for intermittent medication for hypercholesterolemia. There was no history of diabetes mellitus, hypertension, lung disease or heart disease. There was no sinusitis or ear infection. Her blood pressure was 110/70 mmHg, pulse rate was 74/min, body temperature was 36.5°C, and her respiration rate was 20 breaths/min. Hb/Hct was 14.5 g/dL/41.6%. Erythrocyte sedimentation rate was 54 mm/h and C-reactive protein (CRP) was 0.22 mg/L. Arterial blood gas analysis revealed a pH of 7.425, pCO2 of 42.5 mmHg, pO2 of 90.4 mmHg, and an HCO3 of 22.2 mmoL/L on room air. Total cholesterol was 249 mg/dL and LDL-cholesterol was 172 mg/dL. Blood glucose was 117 mg/dL. Thyroid hormone and pulmonary function tests were all within normal range. Echocardiography showed normal global left ventricular systolic function. Brain computed tomography (CT) showed a mass with perilesional edema on the right frontal lobe. Brain magnetic resonance (MR) imaging revealed a 4×3-cm ring enhanced mass in the right frontal lobe, which was associated with severe edema and midline shifting to the left side (Fig. 1). We performed a craniotomy and abscess removal. Bacteriological culture proved negative. A chest x ray showed nodular infiltrations on the right mid-lung zone and the left upper and lower lung zones. A chest CT showed multiple pulmonary AVFs. Therapeutic embolization was performed (Fig. 2) and antibiotics were maintained for 8 weeks. Her past history gave no indication of exertional dyspnea or episodes of hemoptysis, melena, hematemesis, epistaxis or hematuria that might suggest underlying HHT.
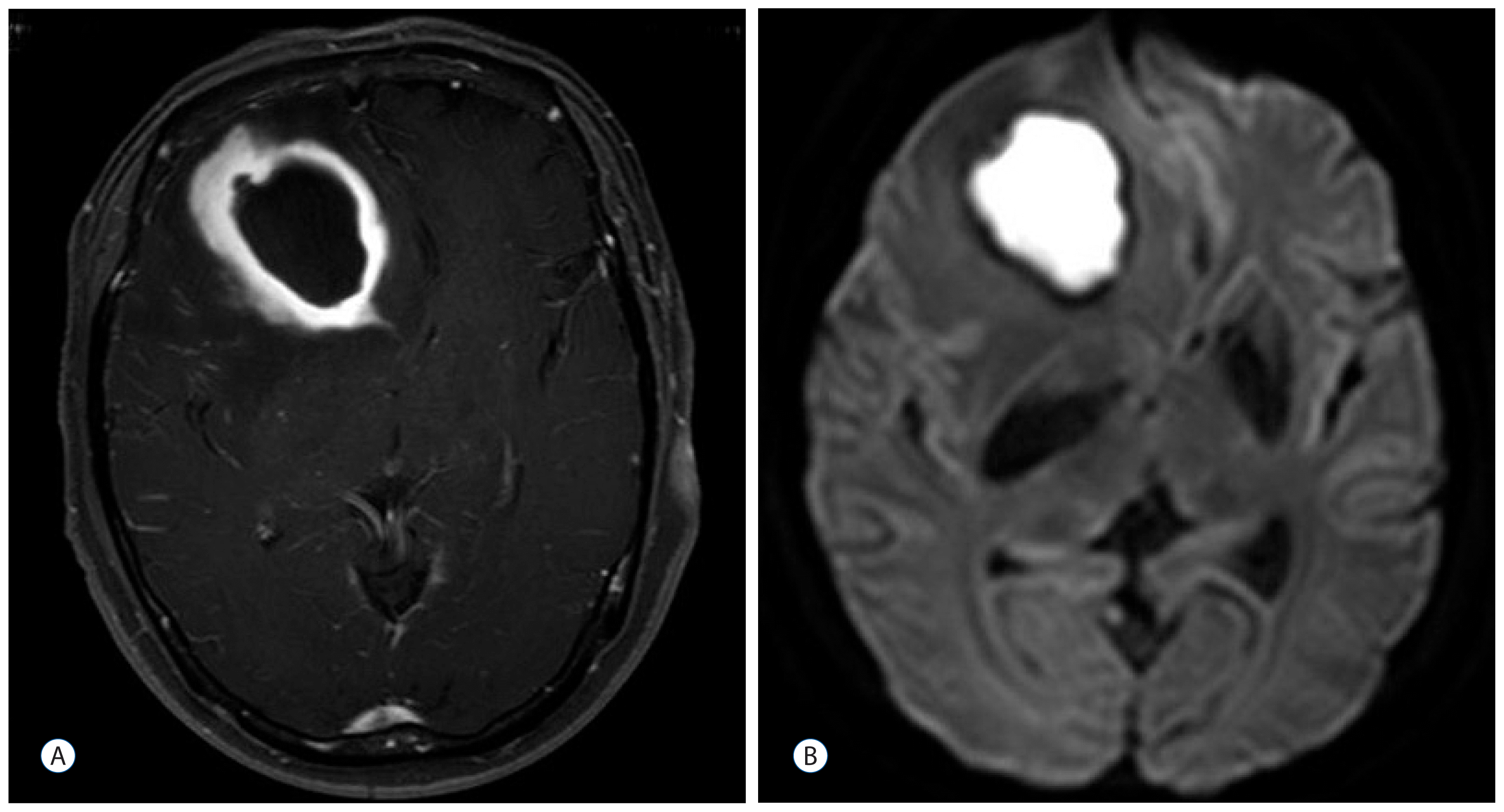
Brain magnetic resonance imaging of case 1. A 4×3-cm ring-enhanced mass in the right frontal lobe, which was associated with severe edema and midline shifting to the left side (A). Diffusion restriction of the enhancing area suggests that the lesion is an abscess (B).
Case 2
A 45-year-old woman presented with a 7-day history of a progressive left hemiparesis. She had no remarkable past medical history or family history. On admission, blood tests showed a Hb of 13.4 g/dL, an Hct of 39.6%, a white blood cell (WBC) count of 6290 cells/μL, and a CRP elevated to 2.62 mg/L. Liver function tests were within normal range. Blood glucose was 101 mg/dL. There were no systemic or focal infection signs. Diffusion MR imaging showed a diffusion-restricted ovoid mass on the right motor and sensory cortex measuring 1.5×0.9 cm that was surrounded by diffuse vasogenic perilesional edema (Fig. 3). MR imaging and MR spectroscopy revealed an enhancing lesion involving the right motor and sensory cortex and increased lactate/lipid complex, which was compatible with a brain abscess. A chest x ray did not suggest underlying lung disease. However, a chest CT revealed a pulmonary AVF in the right upper lung (Fig. 4). The brain abscess progressed despite treatment with vancomycin and ceftriaxone, so it was removed via craniotomy and the pulmonary AVF was embolized. Bacteriological culture of the pus revealed no growth. Additional examinations did not show any evidence of HHT.
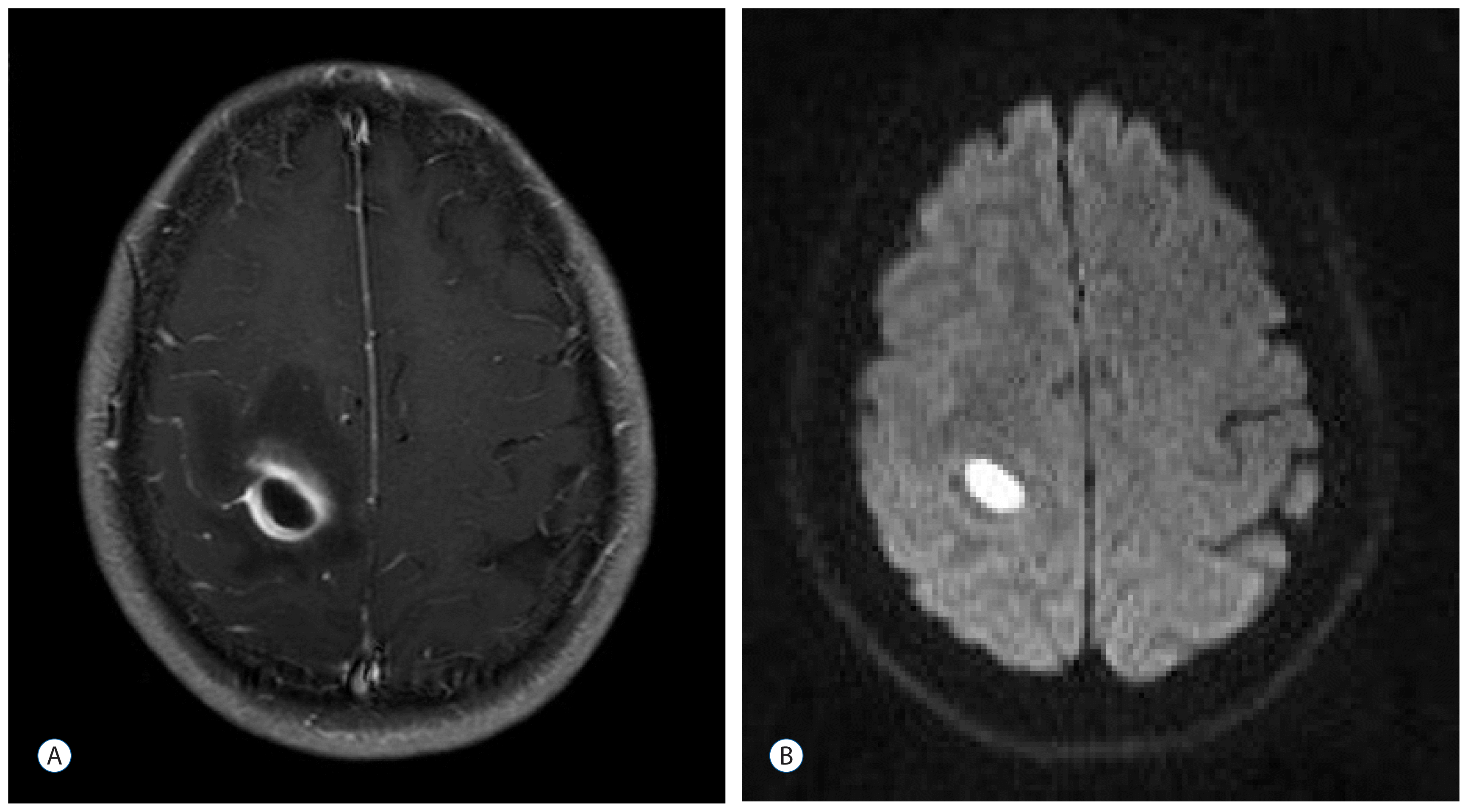
A 45-year-old woman (case 2) presented with a 7-day history of a progressive left hemiparesis. Enhanced brain magnetic resonance (MR) imaging shows a well-enhancing ovoid mass on the right motor and sensory cortex measuring 1.5×0.9 cm (A). Diffusion weighted MR reveals restriction of the mass (B).
DISCUSSION
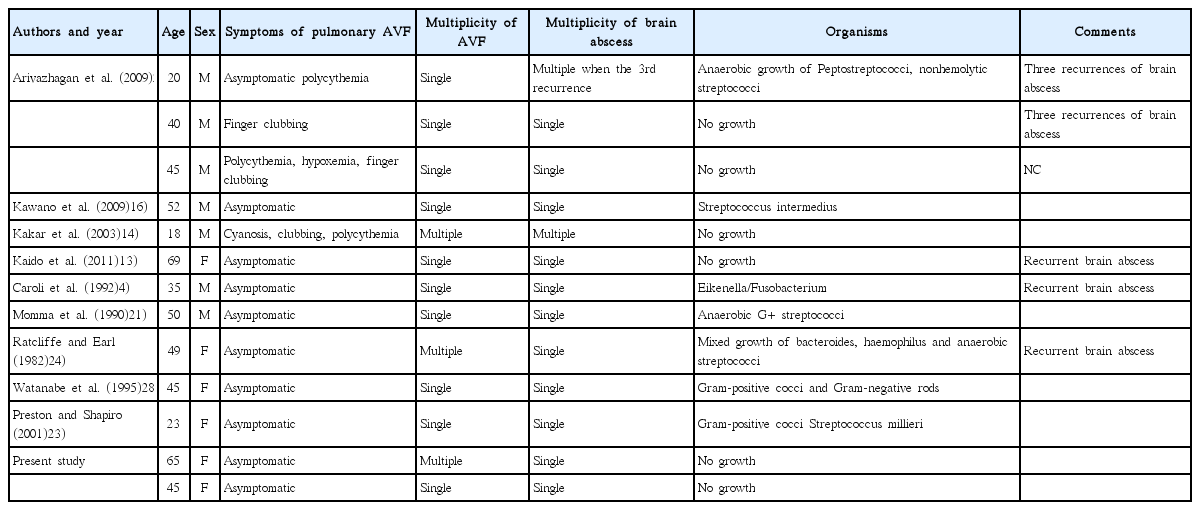
Cryptogenic brain abscesses can occur due to rare diseases that are not addressed in routine clinical practice. Congenital cyanotic heart disease3), patent foramen ovale12,15), thoracic infection19) or asymptomatic dental infections7) are commonly associated with cerebral abscesses. Idiopathic asymptomatic pulmonary AVFs are also a cause of brain abscess, especially recurrent brain abscess (Table 1).
The incidence of idiopathic pulmonary AVF-related CNS complications is between 19 and 59 %11,25,29). Associated neurological events included migraine, transient ischemic attack, stroke, abscess, and seizure17,26,30). The incidence of brain abscess in patients with a pulmonary AVF is around 1 to 5%8).
The most likely mechanism for these neurological events is a paradoxical embolism across the pulmonary AVF or across a coexisting cerebral arteriovenous malformation in patients with HHT17). The pulmonary capillary bed acts as a filter that removes small thrombi and bacteria as they enter the bloodstream, even during daily activities such as oral hygiene. In a pulmonary AVF, the pulmonary capillary bed is bypassed, providing a direct right to left shunt that depends on the diameter of the feeding artery. As a consequence, patients may develop paradoxical emboli that present as a transient ischemic attack, stroke, or brain abscess5).
The fundamental defect that we found was a right-to-left shunt from the pulmonary artery to the pulmonary vein, and the degree of shunting determines the clinical effects. If shunting is minimal, cyanotic symptoms are usually absent. If the right-to-left shunt is greater than 20% of the systemic cardiac output, or if there is reduction of hemoglobin by more than 50 g/L, the patient will have obvious cyanosis, clubbing, and polycythemia17). The characteristic findings of cyanosis, clubbing and an extra-cardiac murmur do not always accompany pulmonary AVF, and diagnosis may be difficult. Asymptomatic patients are common and account for 13% to 56% of patients8,9,30). An absence of symptoms does not preclude diagnosis of pulmonary AVF. A review of the Mayo Clinic experience suggested a morbidity of 26–33% and mortality of 8–16% in untreated patients with pulmonary AVF26). The international guidelines for the management of pulmonary AVF in HHT recommends that treatments should be applied to all adults with AVFs and children with symptomatic pulmonary AVFs. The decision to treat in asymptomatic children (no dyspnea, no exercise intolerance, no growth delay, no cyanosis or clubbing, no previous complication) should be made on a case-by-case basis. The selection of pulmonary AVFs for embolization is based on feeding artery diameter, generally 3 mm or greater10).
A literature review (Table 1) indicates that idiopathic pulmonary AVFs can cause brain abscess in patients as young as 18 years old. Based on these reported series, 10 of 13 patients did not have pulmonary AVF-related symptoms until the brain abscess developed. Five suffered recurrent brain abscesses. Idiopathic pulmonary AVFs are more frequently solitary, around 80%, compared with that of HHT (<40%)30). It means idiopathic pulmonary AVFs are less likely to be associated with large right-to-left shunt, this might in part explain why patients with idiopathic PAVMs might have a lower frequency of cyanosis and polycythemia. Also, previous HHT series have shown an association between number of pulmonary AVFs and cerebral abscess risk22). This might explain why idiopathic pulmonary AVFs are associated with a lower frequency of cerebral abscess than in HHT.
The organisms in the brain abscess were not consistent but most frequently isolated ones were streptococci genus. 6 cases out of 13 did not reveal an organism at all.
If young adults without a premorbid history present with a brain abscess, pulmonary problems must be evaluated.
CONCLUSION
This report highlights the need to consider pulmonary AVF as an etiology of cerebral abscess when routine investigations fail to detect a source. Diagnosis can be confirmed through thoracic CT or pulmonary angiography.