High-Resolution Magnetic Resonance Imaging of Intracranial Vertebral Artery Dissecting Aneurysm for Planning of Endovascular Treatment
Article information
Abstract
The equipment and techniques associated with magnetic resonance imaging (MRI) have rapidly evolved. The development of 3.0 Tesla MRI has enabled high-resolution imaging of the intracranial vessel wall. High-resolution MRI (HRMRI) can yield excellent visualization of both the arterial wall and lumen, thus facilitating the detection of the primary and secondary features of intracranial arterial dissection. In the present report, we describe the manner in which HRMRI affected our endovascular treatment planning strategy in 2 cases with unruptured intracranial vertebral artery dissection aneurysm. HRMRI provides further information about the vessel wall and the lumen of the unruptured intracranial vertebral artery dissecting aneurysm, which was treated by an endovascular approach in the 2 current cases.
INTRODUCTION
The natural history of intracranial vertebral artery dissecting aneurysm (VADA) is unclear, and the evolution of this condition may vary1316). Moreover, the management of VADA remains controversial and lacks a standard protocol1210). In particular, the treatment of unruptured intracranial VADA is unclear, which may be due to the lack of sufficient information. Although unruptured VADA is believed to be benign in nature in most cases12), unruptured intracranial VADA may not be completely safe. Furthermore, if any associated symptoms or changes in imaging findings are noted, treatment should be considered81415). Computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA) have enabled the imaging of the lumen of blood vessels. However, a limitation of these modalities is that they cannot differentiate between vascular pathologies, as different pathologies may exhibit similar luminal defects. However, direct imaging of the blood vessel wall may help discriminate between these pathologies. The equipment and techniques associated with magnetic resonance imaging (MRI) have rapidly evolved. The development of 3.0 Tesla MRI has enabled high-resolution imaging of the intracranial vessel wall. High-resolution MRI (HRMRI) can offer excellent visualization of both the arterial wall and lumen, thus enabling the detection of the primary and secondary features of intracranial arterial dissection. In the present report, we describe the manner in which HRMRI influenced our endovascular treatment planning in 2 cases with unruptured intracranial vertebral artery dissection aneurysm.
CASE REPORT
HRMRI protocol
The patients were placed in a neutral position for imaging with a 3-T MRI scanner (Achieva; Philips Healthcare, Best, The Netherlands) with a 32-channel head. Three-dimensional (3D) time-of-flight angiography (TOF) of the intracranial region was performed. The intracranial VADA that was identified by 3D TOF MRA, was subsequently assessed with HRMRI. A 3D MRA localizer was used to ensure that the cross-sectional images were perpendicular to the VADA. A 3D isotropic proton density (PD)-weighted turbo spin echo (TSE) sequence was performed in the axial plane. Oblique coronal and oblique sagittal multiplanar reconstruction was performed, with a slice thickness of 1.0 mm. PD-weighted, T1-weighted, and gadolinium-enhanced T1-weighted TSE sequence imaging were performed in the oblique sagittal or coronal plane. The parameters for the imaging sequences were as follows : 1) 3D PD TSE imaging [TR/TE, 2000/30.7 ms; field of view (FOV), 18 cm; matrix size, 300×300; slice thickness, 0.6 mm; number of excitations (NEX), 1]; 2) PD TSE imaging (TR/TE, 2000/30.1 ms; FOV, 10 cm; matrix size, 200×200; slice thickness, 2 mm; NEX, 2); 3) T1 TSE imaging (TR/TE, 1000/7.7 ms; FOV, 10 cm; matrix size, 200×200; slice thickness, 2 mm; NEX, 3); and 4) gadolinium-enhanced T1 TSE imaging (TR/TE, 1000/7.7 ms; FOV, 10 cm; matrix size, 200×200; slice thickness, 2 mm; NEX, 4).
Case 1
A 49-year-old man presented with a sudden occipital headache. An unruptured intracranial VADA was detected on MRA and DSA (Fig. 1A, B). Thereafter, HRMRI was performed. A pseudolumen (to the proximal vessel), intimal flap, and intramural hematoma were detected on PD sequence imaging. Furthermore, the ostium of the branching vessel (anterior spinal artery and posterior inferior cerebellar artery) was detected at the vertebral artery, distal to the VADA. The ostia were not related to the VADA (Fig. 1C, D). The length of the hidden pseudolumen was 9 mm (Fig. 1E, F). Double-stenting was performed, using an Enterprise stent (Enterprise; Codman Neurovascular/Johnson & Johnson, Raynham, MA, USA) with a size of 4.5×28 mm. As the deployed stent did not cover the whole segment of the VADA, a second stent was introduced at the proximal segment to cover the inlet of the VADA (Fig. 1G). No intervention-related complications were noted. The patient has been receiving outpatient treatment and is free of any symptoms.
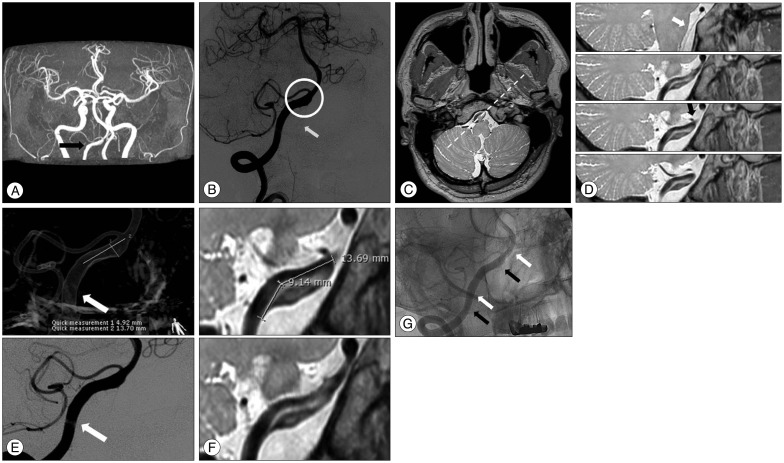
A : Magnetic resonance angiography (time of flight image) indicates aneurysmal dilatation at the intradural segment (V4) of the right vertebral artery (arrow indicates the aneurysm). B : Digital subtraction angiography confirms the presence of aneurysmal dilatation at the same level (the hallow indicates the aneurysm). The anterior spinal artery is incorporated into the aneurysmal sac (the arrow indicates the anterior spinal artery). C : An axial image with a proton-density (PD) sequence at the level of the vertebral artery dissecting aneurysm (VADA) obtained through high-resolution magnetic resonance imaging (HRMRI). D : The PD sequence along the dotted line on C shows a long pseudolumen and intimal flap. The ostia of the anterior spinal artery and posterior inferior cerebellar artery are detected at the vertebral artery distal to the VADA (the white arrow indicates the ostium of the anterior spinal artery, whereas the black arrow indicates the ostium of the posterior inferior cerebellar artery). E : Three-dimensional rotation angiography and digital subtraction angiography of the right vertebral artery show a dissecting aneurysm (arrows indicate the real inlet of the dissecting segment). F : HRMRI shows the hidden pseudolumen and intramural hematoma. The length of the hidden pseudolumen was 9 mm, compared with that noted on digital subtraction angiography. G : Double-stenting was performed (white arrows indicate the distal markers of the first deployed stent, whereas black arrows indicate the distal markers of the second deployed stent).
Case 2
A 37-year-old man presented with a sudden occipital headache. An unruptured intracranial VADA was detected on CTA and DSA. (Fig. 2A, B) Subsequently, HRMRI was performed. HRMRI indicated a hidden pseudolumen and intramural hematoma at the vertebral artery proximal to the VADA, which could not be detected on DSA (Fig. 2C). The length of the hidden pseudolumen was 7 mm (Fig. 2D, E). Furthermore, the hidden pseudolumen was located on the opposite side of the dissecting aneurysm that was noted on DSA. Stent-assisted coiling was then performed, using an Enterprise stent with a size of 4.5×28 mm and 9 detachable coils (length, 36 cm) (Fig. 2F). No intervention-related complications were noted. The patient has been receiving outpatient treatment, and is free of any symptoms.
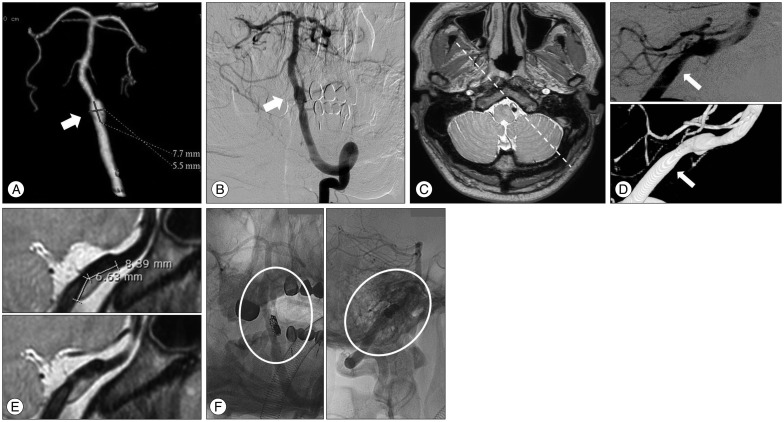
A : Computed tomography angiography indicates a dissecting aneurysm at the intradural segment of the left vertebral artery. The length of the dilated segment was 7.5 mm (the arrow indicates the aneurysm). B : Digital subtraction angiography confirms the presence of a dissecting aneurysm at the same level with proximal stenosis (the arrow indicates the aneurysm). C : An axial image with a proton-density sequence at the level of the vertebral artery dissecting aneurysm obtained through high-resolution magnetic resonance imaging (HRMRI). D : Three-dimensional rotation angiography and digital subtraction angiography of the left vertebral artery show a dissecting aneurysm (the arrow indicates the real inlet of the dissecting segment). E : HRMRI shows the hidden pseudolumen. The length of the hidden pseudolumen was 7 mm, compared with that noted on digital subtraction angiography. Furthermore, the hidden pseudolumen and intramural hematoma were located on the opposite side of the dissecting aneurysm that was identified on angiography. F : Angiography after stent-assisted coiling in the working angle indicates a relatively well-preserved vertebral artery.
DISCUSSION
HRMRI can provide a considerable amount of information for the differential diagnosis of atherosclerotic disease, CNS inflammatory disease, dissection, aneurysms, moyamoya disease, and others51819). HRMRI has recently emerged as a potentially useful technique for imaging of atherosclerotic plaques in the intracranial artery4). The identification of dissection in the intracranial artery is also being actively investigated. It has been shown that HRMRI can help assess the region beyond the vessel lumen, thus providing direct visualization of the dissection in the arterial wall3615). The typical features of arterial dissection on HRMRI include eccentric wall thickening, diffuse wall enhancement, and good visualization of false lumen with intramural hematoma19). The lumen of the vessel and intimal flap are well visualized on T2 and PD sequences, whereas hyperintense intramural hematoma in the arterial wall is well visualized on T1 and magnetization-prepared rapid acquisition gradient-echo sequences due to the presence of methemoglobin91819). HRMRI could help in clearly differentiating between intramural hematoma and intraluminal thrombus620). Furthermore, the investigation of the natural history may possible with HRMRI.
With regard to the clinical benefits of HRMRI, it provides detailed information of hidden structures such as intimal flap, inlet of the false lumen, size of the intramural hematoma, and ostium of small branching vessel20). The presence of pathological features such as intramural hematoma on the wall may serve as evidence that the aneurysm is unstable. In such cases, close observation or consideration of treatment may be mandatory111517). HRMRI can obtain this vital information. In the present cases, HRMRI provided detailed information on the actual length and direction of the VADA; presence of a pseudolumen, intramural hematoma, and inlet of the pseudolumen; and the relationship between the vertebral artery and the branching vessel such as the anterior spinal artery and posterior inferior cerebellar artery. This information cannot be obtained with DSA only. Based on this information, we can determine the locations that should be avoided and the exact segment that should be covered with stent. Furthermore, detailed information may help to reduce procedural complications during navigating, stenting, and coiling7).
Nevertheless, there are certain limitations of this technique. HRMRI is usually performed in addition to baseline imaging, and hence, the scan time is longer. Moreover, HRMRI of the intracranial vessel requires the use of a 3.0 Tesla MRI. The long scan duration may be a burden for neurologically unstable patients. However, such limitations will gradually be overcome through further development of the MRI machine and evolution of MRI techniques.
CONCLUSION
HRMRI provides further information about the vessel wall and the lumen of the unruptured intracranial VADA, which was treated by an endovascular approach in the 2 current cases.