Malignant Rhabdoid Tumor of the Kidney and Spine in an Infant
Article information
Abstract
Rhabdoid tumor of the kidney (RTK) is a rare malignancy in infancy. Central nervous system involvement in RTK is already known. However, solitary spinal metastasis in RTK has been hardly reported. The authors report a case of metastatic RTK to spine causing paraplegia in an 8-month-old girl. Since the patient was young, the diagnosis of spine metastasis was delayed until paraplegia was seen after radical nephrectomy. Thorough neurological examination should be performed for early diagnosis of spinal metastasis in young patients with RTK. If there are any abnormal signs in neurologic examination, magnetic resonance images of brain and spine are recommended.
INTRODUCTION
Rhabdoid tumor of the kidney (RTK) is a rare and highly malignant neoplasm of infancy3). RTK constitutes only 1.7% of all renal tumors15), but has a strong tendency for early metastasis to distant region2). According to previous studies, 22-28% of the patients had metastatic disease at the initial diagnosis, and lung metastases were predominant13,14). RTK is known to be associated with primary or metastatic brain tumors3), and previous studies reported that 10-21% of RTK patients had central nervous system (CNS) involvements11,13). Since there is a possibility of tumor spread through CSF pathway, CNS involvement is considered as a poor prognostic factor1). However, most of CNS involvements in previous studies described brain involvement. The rate of solitary spinal metastasis was hardly reported. The symptoms of brain involvement are associated with raised intracranial pressure such as vomiting and a decrease level of consciousness6). However, since the symptoms of spinal involvements are inability to walk, pain, or sphincteric deficit, it is difficult to detect spinal metastasis in young patients who are unable to express their symptoms.
In the current report, we present a case of an 8-month-old girl who had RTK with solitary spine metastasis. Due to her young age, the diagnosis of spine metastasis was delayed until paraplegia was seen after radical nephrectomy.
CASE REPORT
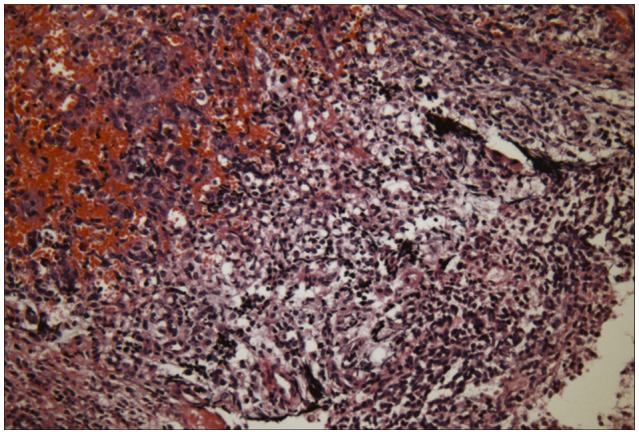
An 8-month-old girl without specific past medical history was admitted to the hospital due to nausea and diarrhea. On physical examination, a firm mass was detected in the left upper quadrant of the abdomen. Abdominal computed tomography (CT) revealed a 6.8×5.8×5.3 cm sized left renal mass without lymph node enlargement (Fig. 1). There were no suspicious metastatic lesions in chest CT. Laboratory data showed leukocytosis. Radical nephrectomy with lymph nodes dissection was performed. Gross examination revealed a well circumscribed, ovoid, firm mass in the lower pole of the kidney (Fig. 2A). The mass was abutted on the renal capsule, and the surface of the mass was whitish gray with multifocal hemorrhages. The remaining renal parenchyma was unremarkable. Microscopic findings revealed that the tumor was a highly cellular neoplasm composed of tumor cells of varying size with prominent nucleoli and abundant cytoplasm (Fig. 2B). Histological examination was consistent with the diagnosis of malignant rhabdoid tumor. Immunohistochemistry showed loss of INI1 protein staining (Fig. 2C).

Histologic and immunostain appearance. Cut section of the kidney reveals a well circumscribed, ovoid, firm mass in the lower pole of the kidney (A). Histology showed the characteristic findings of rhabdoid tumor including a highly cellular neoplasm composed of tumor cells of varying size with prominent nucleoli and abundant cytoplasm (B, hematoxylin-eosin stain, ×400). Immunostains showed negative staining for INI1 (C).
One week after the operation, weakness of both lower extremities and voiding failure were noted. On neurological examination, she failed to respond to pain in both legs. Brain magnetic resonance imaging (MRI) did not show evidence of metastatic disease, but lumbar MRI revealed a 6.5×1 cm sized intradural extramedullary tubular mass with heterogeneous enhancement at L1-S1 level (Fig. 3). On the next day from onset of symptoms, laminoplastic laminotomy was performed by a neurosurgeon to remove the tumor and decompress spinal cord. Conus medullaris was displaced by the hypervascular tumor with yellowish color, however the upper margin of the tumor was clear (Fig. 4A). The tumor was densely adhered to the left side of L5 nerve root. Near total removal of the tumor was performed using cavitron ultrasonic surgical aspirator, and there was no root injury during the surgery (Fig. 4B). The pathologic findings were consistent with metastatic malignant rhabdoid tumor (Fig. 5). After the surgery, the motor power of lower extremity was returned. She received adjuvant radiotherapy for 2 weeks and 13 cycles of chemotherapy (vincristine, doxorubicin, and cyclophosphamide) for 3 months postoperatively. The patient showed general weakness, seizure and changes in mental status during the chemotherapy. Leptomeningeal metastasis with hydrocephalus was noted in brain MRI. A ventriculoperitoneal shunt was placed. However, she did not clinically improve and died 6 months after the surgery.

The T1-weighted enhancing lumbar MRI shows a 6.5×1 cm sized intradural extramedullary tubular mass with heterogeneous enhancement at L1-S1 level.
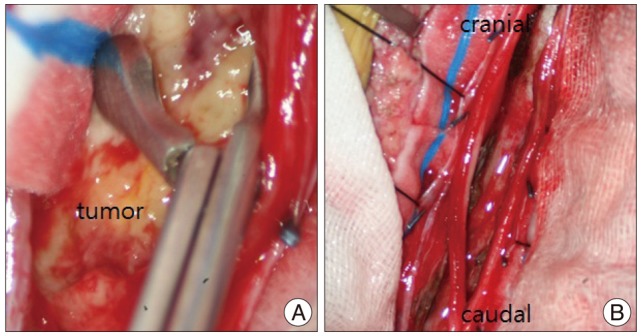
A : Intraoperative photography shows the tumor with pale yellowish color. B : Final photography after removal of the tumor shows nerve root.
DISCUSSION
RTK is a rare and highly malignant neoplasm of infancy3). Once regarded as a sarcomatous variant of Wilms' tumor, it is now regarded as a distinct entity unrelated to Wilms' tumor7). Incidence of metastatic disease in RTK (22-28%) is more frequent than Wilms' tumor, and CNS involvement is reported as 10-21%11,13,14). CNS involvement in RTK patients also includes synchronous intracranial neoplasm like pineoblastoma, medulloblastoma, primitive neuroectodermal tumor, astrocytomy, ependymoma, and germ cell tumor as well as brain metastasis3). Thus, intracranial imaging in RTK patients is recommended at diagnosis.
The frequency of CNS involvement in RTK patients was variously reported in previous studies. National Wilms' Tumor Study (NWTS) reported a high rate of CNS involvement with an incident rate of 21%13). In the International Society of Pediatric Oncology, only 2.8% of the RTK patients had CNS involvement because CNS evaluation was not a mandatory diagnostic procedure14). Reinhard et al.10) reported that only 1 patient had synchronous brain lesion among 70 patients of rhabdoid tumor (32 RTK patients). However, these studies only reported either CNS involvement or brain lesion, and failed to evaluate the rate of solitary spinal metastasis. To our knowledge, there is no case report about RTK patients with spinal metastasis causing paraplegia. Herein, we described a RTK patient who had solitary intradural extramedullary metastasis. In previous studies, MRI was reported as a highly accurate diagnostic modality to detect spinal cord tumor, and the sensitivity of MRI was reported as 100%9,12). The sensitivities of CT scan were 93% and 73% for patients with extradural and intradural tumors, respectively. In the present case, spinal metastasis was detected only in MRI, not in CT scan. Usually, back pain may be a leading clinical complaint from spinal cord tumor5). However, since the incidence of spinal cord tumor is very rare, physicians may start conservative management for back pain if abdomino-pelvic CT or radiography is negative. Only 7 cases of primary malignant rhabdoid tumor of the spine have been reported in the literature4), and solitary spinal metastasis of RTK is extremely uncommon as mentioned above. These may contribute to the delayed diagnosis of spinal involvement. Although spinal MRI could be not performed in all RTK patients, thorough neurological examination might aid early detection of spinal involvement, especially in patients who are too young to express their symptoms such as back pain or sensory/motor loss of lower extremity. NWTS reported that RTK patients younger than 1 year of age had poor prognosis older than 1 year of age13). Furthermore, among 30 patients who had CNS involvement, 26 patients (86%) were younger than 1 year of age at diagnosis. Thus, the evaluation of CNS involvement including spinal MRI or neurological examination should be considered especially in young patients (<1 year of age).
With early recognition, spinal cord compression can be prevented, minimized, or possibly reversed12). However, failure to recognize the condition and its serious nature, in addition of a lack of awareness of the importance of early referral for treatment can result in irreversible paralysis. Tantawy et al.12) reported that it took 2-22 months to diagnosis malignancy in spine after the first appearance of symptoms of spinal cord compression (inability to walk, pain, or sphincteric deficits). In adult patients with spinal cord compression due to metastatic spinal tumor, clinical outcome in terms of ambulatory recovery was promising after decompressive surgery, especially in patients with preoperative grade III of lower extremity motor power8). However, studies evaluating motor power of infants after decompressive surgery hardly exist. Considering the median age of RTK patients is 11 months13), about half of the patients seem to be unable to walk at diagnosis. In these patients, it may be difficult to predict the return of the lower extremity motor power after decompressive surgery. RTK is considered one of the most aggressive malignant solid tumors, and the overall survival is reported to be 20-25%13). Thus, early diagnosis and intervention is necessary for preventing sequela such as spinal cord compression in survivors. In the present case, the motor power of lower extremity was returned after laminotomy, although the patient failed to survive. Although oncological outcome is the most important, permanent sequela after treatment should be always considered in infants because it affects quality of life.
CONCLUSION
RTK with solitary spinal metastasis is very rare. Since RTK prevalent in young children, the early detection of spinal metastasis may be difficult until paraplegia is seen. Thorough neurological examination at diagnosis to detect spinal metastasis should be considered in young RTK patients.