Isolated Lateral Sinus Thrombosis Presenting as Cerebellar Infarction in a Patient with Iron Deficiency Anemia
Article information
Abstract
As a rare cerebrovascular disease, cerebral venous thrombosis (CVT) is caused by various conditions including trauma, infection, oral contraceptive, cancer and hematologic disorders. However, iron deficiency anemia is not a common cause for CVT in adult. Posterior fossa infarction following CVT is not well demonstrated because posterior fossa has abundant collateral vessels. Here, we report a case of a 55-year-old man who was admitted with complaints of headache, nausea, and mild dizziness. The patient was diagnosed with isolated lateral sinus thrombosis presenting as cerebellar infarction. Laboratory findings revealed normocytic normochromic anemia due to iron deficiency, and the patient's symptoms were improved after iron supplementation.
INTRODUCTION
Cerebral venous thrombosis (CVT) is an infrequent cerebrovascular disease encompassing thrombosis of the cerebral veins and the dural sinuses2,12). CVT is characterized by a wide spectrum of clinical presentations which depend on the site, extent, and progression of thrombosis2,3,11,12). For this reason, it has often been difficult to distinguish CVT from central nervous system neoplasms, infections and neurodegenerative conditions10,12).
CVT is caused by various conditions including infection, oral contraceptive or steroid use, cancer, dehydration, hematologic disorder and trauma1,5,12). For the management of the CVT patient, therefore, it should be necessary to determine underlying cause as well as symptomatic treatment. Most CVTs occur at supratentorial parenchymal regions because there are abundant collateral vessels in the posterior fossa2,7). Hence, cerebellar infarction following CVT associated with iron-deficiency anemia (IDA) is not well demonstrated. In the presenting study, we report a case of cerebellar infarction which resulted from IDA induced isolated lateral sinus thrombosis.
CASE REPORT
A 55-year-old man was admitted to the emergency department with a 15-day history of headache, nausea, and mild dizziness. The patient had no medication history and no underlying diseases including cancer, infection and hematologic disorder. In addition, he had not suffered from trauma. The patient was alert mentality and there were no abnormal neurologic findings for cranial nerves, muscle strength and deep tendon reflex. The cerebellar function test, however, showed a mild disturbance in tandem gait.
Vital signs at admission showed a body temperature 36.7℃, a blood pressure of 100/60 mm Hg, and a heart rate of 70 beats/minute. Laboratory findings were as follows : hemoglobin 7.2 g/dL, hematocrit 24.8%, white blood cell count 4600/µL, platelet count 130000/µL, erythrocyte sedimentation rate 12 mm/hr, and C-reactive protein 1.447 mg/L. Coagulation profiles demonstrated a prothrombin time of 82%, an international normalized ratio of 1.13, an activated partial thromboplastin time of 28.8 seconds, and a bleeding time of 3.00. The following laboratory examinations were done to evaluate his anemia : total iron-binding capacity 324 ug/dL, serum iron concentration 54 ug/dL, vitamin B12 294 pg/mL, and folate 11.4 ng/mL. Peripheral blood smear test showed normocytic normochromic anemia with anisopoikilocytosis. These findings were consistent with IDA. Magnetic resonance image (MRI) of the brain showed high signal intensity on the T2 weighted/FLAIR image and low signal intensity on the T1 weighted image of the right cerebellar hemisphere compressing the fourth ventricle (Fig. 1A, B). There was multifocal petechial hemorrhage and irregular enhancement in the right cerebellar hemisphere on the gadolinium-enhanced T1 weighted image (Fig. 1C). In addition, there was a high signal lesion on the junction of the right transverse sinus and proximal sigmoid sinus on the T2 weighted image and FLAIR image. To determine the malignant neoplasm, PET-CT scans and tumor markers were examined. PET-CT showed no hypermetabolic lesion in the cerebellar parenchyme. However, diffuse hypermetabolism was detected in bone marrow, which was probably a reactive change due to the patient's anemic condition. Tumor markers were normal : AFP 1.86 ng/mL, CEA 0.659 ng/mL, CA 19-9 1.32 U/mL, CA125 10.01 U/mL, and PSA 0.509 ng/mL. Cerebral angiography demonstrated a filling defect in the right side transverse sinus and proximal sigmoid sinus in the venous phase (Fig. 2). Some venous congestion along with collateral vessels was also developed in the right cerebellar region.
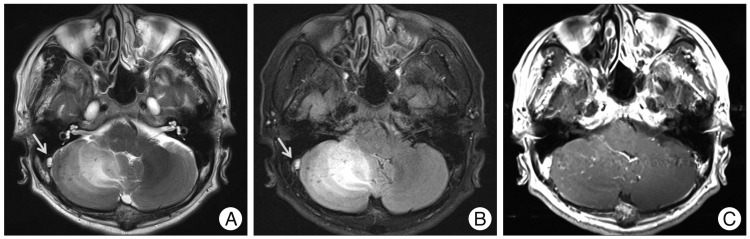
Axial T2-weighted (A) and FLAIR (B) magnetic resonance imaging scan reveal high signal intensity in the right cerebellar hemisphere compressing the fourth ventricle. In addition, there is high signal intensity at the junction of the right transverse sinus and proximal sigmoid sinus, probably due to a thrombus (arrow). Gadolinium-enhanced T1 weighted image demonstrates petechial hemorrhage and irregular enhancement (C).
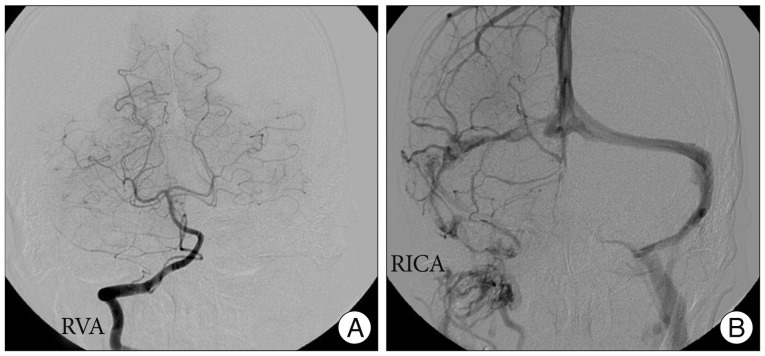
A : Right vertebral angiography does not reveal abnormal findings. B : In the venous phase, however, there are multiple filling defects of the right transverse sinus and proximal sigmoid sinus.
There were negative findings for antinuclear antibody, lupus anticoagulant, antiphospholipid antibody IgG/M, anticardiolipin antibody IgG/M, anti beta2 GPI antibody IgG/M, and PNH flowcytometry. In addition, no abnormality was found in the level of protein C and protein S : protein C antigen 55% and protein S antigen 52%. Thyroid function test was done as follows : T3 87.2 ng/dL, TSH 5.66 uIU/mL, and fT4 1.08 ng/dL.
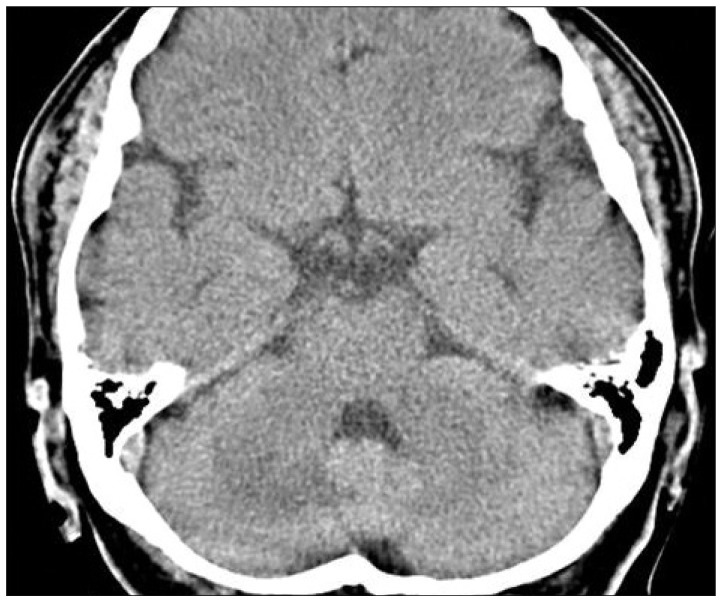
The patient received mannitol to control the increased intracranial pressure, and ferrous sulfate 256 mg (80 mg as iron) was given daily for the IDA. For preventive purpose, aspirin and clopidogrel were also prescribed. Cerebellar edema was markedly improved on the follow-up CT scan (Fig. 3). Laboratory findings were also improved : hemoglobin 8.6 g/dL and hematocrit 29.0%. The patient was discharged with no neurologic deficits. After 1 month, the patient had no symptoms and anemia was completely recovered : hemoglobin 11.3 g/dL and hematocrit 35.8%.
DISCUSSION
The most frequent sites of CVT are both the superior sagittal sinus and the lateral sinus, and only 10% of CVT involves the isolated lateral sinus2). Thromobosis of these sinuses usually causes infarction and/or hemorrhage of the supratentorial region2,8,11). Damak et al.2) demonstrated that 80% of isolated lateral sinus thromboses revealed a parenchymal lesion in the temporal lobe. The case of cerebellar infarction limited to the posterior fossa resulting from venous sinus thrombosis is very rare because there is abundant collateral venous drainage of posterior fossa structures2,8,11). However, deficiency of the collateral venous drainage pathway may induce a localized cerebellar infarction as seen in the present case. Together with these findings, isolated lateral sinus thrombosis should be considered in the differential diagnosis of infarction limited to the cerebellar hemisphere.
Cerebellar infarction associated with CVT seems to be a nonspecific parenchymal feature in radiologic findings. In our case, we found a diffuse swelling of the right cerebellar hemisphere on MR images. There was no definite mass-like enhanced lesion on gadolinium-enhanced MR images. In addition, the cerebellar parenchymal lesion was not consistent with arterial vascular territory, suggesting that it may not be caused by arterial occlusion. However, high signal intensity was noted on the junction of the right transverse sinus and proximal sigmoid sinus on MR images (Fig. 1A, B). It has been known that thrombus in CVT contains methemoglobin and causes high signal intensity on T1- or T2 weighted images on MRI12). Therefore, these findings on MRI may increase the possibility of CVT and suggest cerebral angiographic examination including the venous phase for accurate diagnosis.
In addition to the symptomatic management of CVT, it is also important to determine the cause of CVT to prevent recurrence. It has been known that various causes can induce CVT including dehydration, pregnancy, puerperium, contraceptives, protein C deficiency, mastoiditis, tumor invasion of a venous sinus, vasculitis, head trauma, intracranial and systemic infection1,2,5,7,8,10,11). CVT can occur by IDA1,5). CVT is usually associated with IDA in children, but not in adults1). Sébire et al.13) reported that about 55% of CVT was associated with anemia in children. However, there has been a few of case reports in adults1,5,6,9). Laboratory findings from our case were consistent with IDA, whereas other causative factors for CVT were not identified. The patient also showed diffuse hyperplasia of bone marrow on PET-CT scans, suggesting a reactive change in response to anemia4). Although PET-CT scanning may not provide specific findings, it can be used to detect anemia. Several mechanisms have been proposed to explain the correlation between IDA and cerebral venous thrombosis1,5). Thrombocytosis occurs secondary to IDA and may involve being in the hypercoagulable state. In addition, IDA may also contribute to the hypercoagulable state by affecting flow patterns within the vessels due to reduced red cell deformability and increased viscosity. Therefore, we can conclude that IDA may contribute to the isolated lateral sinus thrombosis followed by cerebellar infarction in the present case. Furthermore, IDA induced CVT did not show any different features from other causes in clinical presentations and sinus involvement1,5,6,9). This is the first case report of isolated lateral sinus thrombosis presenting as cerebellar infarction in a patient with iron deficiency anemia.
Although a few studies have reported about the management of CVT, most of them are controversial12). There are several recommendations for the management of CVT, including initial anticoagulation, mechanical thrombectomy/thrombolysis, antibiotics in infectious conditions, steroids, intracranial pressure control such as mannitol and acetazolamide, and surgical intervention12). In the acute stage of CVT, it is reasonable to perform initial anticoagulation using unfractionated heparin or low molecular weighted heparin12). Endovascular intervention can be considered if neurological deterioration occurs despite intensive anticoagulation12). Surgical management such as decompressive hemicraniectomy may be considered in patients with severe mass effect or intracranial hemorrhage leading to intractable intracranial hypertension12). Oral anticoagulation is recommended to prevent the progression of thrombosis and recurrent thromboembolic events12,14). In our case, the patient had a 15-day history of headache and mild dizziness, and his neurologic deficit was not significant. For these reasons, heparinization or endovascular intervention was not considered. The patient received mannitol to control for his increased intracranial pressure and ferrous sulfate for correction of IDA.
CONCLUSION
IDA should be considered as one of the causative factors for cerebellar infarction associated with CVT.