Totally Ossified Metaplastic Spinal Meningioma
Article information
Abstract
A 61-year-old woman with a very rare case of totally ossified large thoracic spinal metaplastic meningioma, showing progressing myelopathy is presented. Computed tomographic images showed a large totally ossfied intradural round mass occupying the spinal canal on T9-10 level. Magnetic resonance imaging revealed a large T9-10 intradural extramedullary mass that was hypointense to spinal cord on T1- and T2-weighted sequences, partial enhancement was apparent after Gadolinium administration. The spinal cord was severely compressed and displaced toward the right at the level of T9-10. Surgical removal of the tumor was successfully accomplished via the posterior midline approach and the histological diagnosis verified an ossified metaplastic meningioma. The clinical neurological symptoms of patient were improved postoperatively. In this article we discuss the surgical and pathological aspects of rare case of spinal totally ossified metaplastic meningioma.
INTRODUCTION
Spinal meningiomas are the second most common tumors after schwannoma and they represent about represent 25 to 46% of spinal tumors18). They most frequently occur in the thoracic region in middle-aged women9,12,14,16,24). Although the most common calcified intradural spinal tumor is a meningioma, totally ossified cases are uncommon and account for only 1-5% of all spinal meningiomas2,6). A ossified spinal meningioma is more difficult to resect than the usual type of meningioma. Hypointensity in T1 as well as T2-weighted images of MRI should make the surgeon suspicious of calcified condition, which may in some cases complicate tumor resection.
We describe a case of symptomatic totally ossified large spinal metaplastic meningioma at the level of T9-10, and discuss the differential diagnosis of ossified spinal intradural tumor based on neuroimaging and the surgical strategy for the totally calcified and ossified spinal meningioma.
CASE REPORT
A 61-year-old woman had a 1-year history of numbness in right lower extremities that progress bilaterally and ascend to his half lower body below the umbilicus. She also noticed progressive urinary incontinence without weakness in lower extremities. Neurological examination revealed a hypoesthesia to touch and pain from T10 to S2 level. Also, she had positive sign in Romberg test. Both patella and ankle reflexes were hypoactive and the Barbinski signs were bilaterally negative. Computed tomographic images without enhancement show a large hyperdense intradural round mass occupying the whole spinal canal at T9-10 level (Fig. 1). Magnetic resonance imaging revealed a large T9-10 intradural extramedullary mass that was hypointense to spinal cord on T1- and T2-weighted sequences, partial enhancement was apparent after Gd administration (Fig. 2). The spinal cord was severely compressed and displaced toward the right.
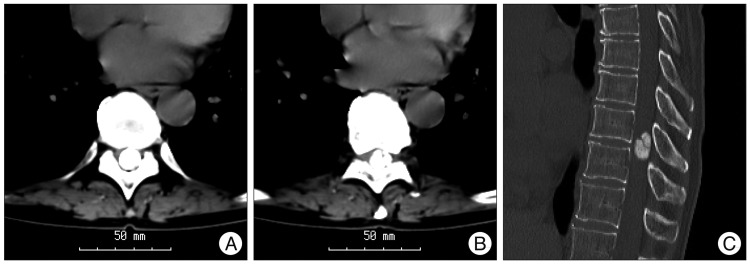
Computed tomographic scan without enhancement show a large totally ossified intradural round mass occupying the whole spinal canal on T9-T10 level. Axial view on T9 (A),10 level (B) and sagittal view (C).
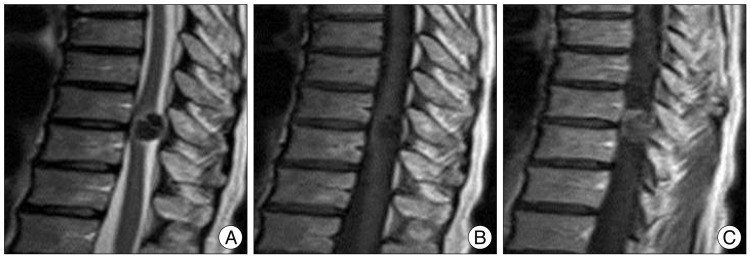
Magnetic resonance image showing the tumor was very low signal intensity on T2-weighted image and mainly low intensity compared to spinal cord on T1-weighted image (A and B). T1-weighted imaging gadolinium showed a round homogenously enhanced tumor and the lower signal intensity of central tumor portions were less enhanced compared with the other portions of the masses (C).
Under general anesthesia, the patient was positioned prone after placement of transcranial muscle motor evoked potentials (Tc-MEPs) and somatosensory evoked potentials (SSEPs) monitoring devices. A T9-10 laminectomy was performed after midline splitting of T9-10 spinous process. The dura mater was exposed and the tumor location has been confirmed at the T9-10 level by ultrasonography. An operative microscope was used during opening the dura, the dura was incised at the midline, exposing a hard and totally ossified, round dural-based extramedullary mass compressing and displacing the spinal cord to the right. We performed tumor debulking with an ultrasonic surgical aspirators. Adhesions between the tumor and the spinal cord were meticulously separated after adequate internal decompression of the tumor (Fig. 3). Surgery proceeded slowly and en block resection of the tumor was performed without significant changes in MEP monitoring. Using the microscissors and microdissector, the tumor and its dural attachment at the left side were removed from the spinal cord. The dural attachment was thoroughly coagulated using bipolar cauterization. A gross-total resection could be achieved with en block manner. Finally, the dura was closed with a Gore-Tex membrane (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) as an intradural sheet to prevent arachnoiditis.
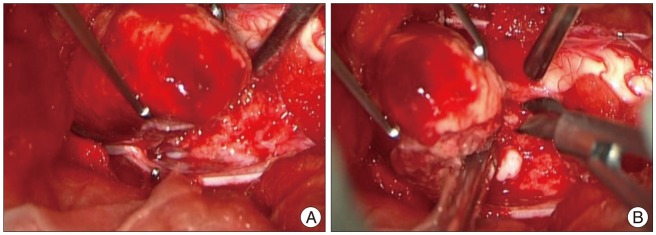
In the totally ossified metaplastic meningioma. It is difficult to find the arachnoid membrane that acts a natural barrier and provides a dissection plane during surgery (A). This tumor has been dissected meticulously through the adhesive peritumoral arachnoid membrane with microsurgical scissor and en-bloc technique (B).
Histopathological findings demonstrated delicate oval nuclei and inconspicuous nucleoli, lightly eosinophilic cytoplasm, proliferate forming cellular whorls. Within the tumor, marked heterotopic ossification occurs without psammoma bodies (Fig. 4). The findings were consistent with spinal metaplastic meningioma. Postoperative MR imaging revealed no evidence of residual tumor. Three days after surgery, the patient had regained full urinary continence, ambulated without an assistive device, and exhibited normal motor strength with residual numbness in her both feet. Prior to discharge from the hospital on postoperative day 31, she could ambulate without a cane and both leg numbness and sensation of pain as well as touch had improved. However, left foot numbness persisted and it caused her hard to go up the stairs for herself. She benefited from a short stay in the inpatient rehabilitation unit.
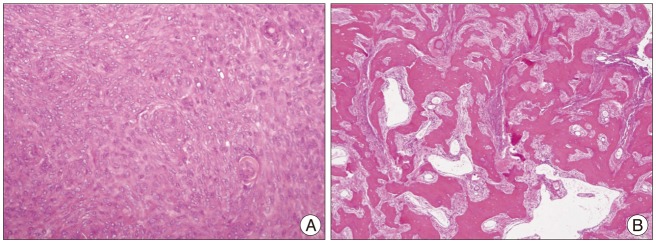
Histologic examination of the initially resected tumor specimen stained with hematoxylin and eosin shows metaplastic (osseous) meningioma. A : The tumor cells which have delicate oval nuclei, inconspicuous nucleoli, and lightly eosinophilic cytoplasm, proliferate forming cellular whorls (×40). B : Within the tumor, marked heterotopic ossification occurs without psammoma bodies (×10).
DISCUSSION
The incidence of intradural tumors in the spine is estimated to be from 3 to 10 per 100000 persons per year, intradural extramedullary tumors account for 2/3 of all intraspinal neoplasms and are mainly represented by meningiomas and schwannomas. Among these spinal intradural tumors, spinal meningiomas are the second common and account for approximately 25-46% of all spinal cord tumors. These tumors occur more frequent in females and peak in the fourth to sixth decade of life10,16).
Spinal meningiomas usually arise intradurally from the arachnoid villi closely related to an emerging nerve root. However, approximately 3-15% of all cases of spinal meningiomas is extradural4,23).
Although spinal meningiomas are not uncommon, calcified cases are rare. A totally calcified spinal meningioma is very rare in spinal lesion1,3,5). As compared to a cranial lesion, the incidence of calcification visible by plain radiography has been reported to be 1-5% of cases1,3,5,7,8,11,17,20,22,23). Because intraspinal spaces are much smaller than intracranial spaces, symptoms may appear more rapidly with intraspinal tumors than with intracranial tumors.
In the present case, on CT scans, intradural extramedullary large masses occupying the whole spinal canal were totally replaced with calcifications with a higher density compared with the adjacent bone marrow density (Fig. 1). The masses showed mainly low intensity compared to spinal cord on T1-weighted image and very low signal intensity on T2-weighted image. T1-weighted imaging gadolinium showed a round homogenously enhanced tumor and the lower signal intensity of central tumor portions were less enhanced compared with the other portions of the masses (Fig. 2).
Calcified or ossified meningiomas usually have adhesion of the tumor to the surrounding nerve tissue. This adhesion makes the surgery more difficult to dissect in surgical resection and may bring more worse surgical outcome than usual meningioma14,20,25). It appears that the functional outcome depends on the location and extent of the tumor. Levy et al.16) reported the different long-term outcomes after calcified or ossified meningioma surgery in calcified 4 cases of 97 spinal meningiomas. Three out of these four patients had a "disastrous surgical outcome". Freidberg7) reported on an ossified meningioma on the ventral thoracic levels and the difficulties in removing it. Also, many authors have reported the various outcomes in calcified or ossified spinal tumors9,19,20).
Usually in the case of the psammomatous meningioma, the adhesion is not problematic. Because the spinal meningioma is extra-arachnoid tumor and the arachnoid membrane acts a natural barrier and provides a dissection plane during surgery. However, in the case of the metaplastic meningioma, it is different from the psammomatous meningioma. In our case, unexpectedly, the arachnoid membrane have been already changed into fibrotic adhesion without arachnoid dissection plane. Although this totally ossified metaplastic meningioma was located posterolaterally to be exposed easily, it was hard to dissect the plane between the adhesive tumor and pia meter (Fig. 3).
Also, ultrasonic surgical aspirator is useful device to remove intradural tumor completely regardless of its size. The large ossified meningioma could be dissected safely from spinal cord after adequate internal decompression of the tumor. We tried to perform tumor debulking with an ultrasonic device set to low power not to damage the spinal cord.
However, ultrasonic surgical aspirator is not useful to remove this severe ossified tumor. Therefore, we tried to dissect the severe ossified tumor meticulously from the pia meter through the adhesive peritumoral arachnoid membrane with microsurgical scissor by cutting peritumoral barrier and extracted it by en-bloc technique (Fig. 4).
During resection of spinal meningiomas, we have used intraoperative neuromonitoring techniques of Tc-MEPs and SSEPs to reduce inducing postoperative neurologic deficits iatrogenically. Adhesions between the tumor and the normal nervous tissue were meticulously separated and the tumor was gross totally resected without Tc-MEPs significant changes. MEP monitoring was highly useful and guided us to manipulate nervous tissue without compromising function.
In the present case, we have not resected the dura, but rather just coagulated it. A resection of the dura was believed to indispensible to reduce the likelihood of recurrence. However, Klekamp and Samii14) reported that the recurrence rate of tumors was independent of management of the dural attachment such as dural resection, duraplasty and coagulation.
Metaplastic meningioma (MM) constitute an uncommon subset of low grade meningiomas that contain a significant component of fat, bone, cartilage or myxoid tissue. Totally ossified spinal metaplastic meningioma is distinctly rare and how metaplastic ossification changes occur is not known. It is also not clear if the ossification process in psammomatous meningiomas and metaplastic meningiomas are significantly different, although they are classified as distinct histologic subtypes. Psammomatous type, histopathologically, is the most common type of the ossified meningiomas. Psammoma bodies are especially frequent in spinal meningiomas23). Approximately 50-90% of spinal meningiomas have psammoma bodies compared with only 10% of the intracranial form21). Camp3) have stated that calcified psammoma bodies can increase radiodensity to such an extent that they are seen on radiographs. Kubota et al.15) ultrastructurally investigated the initial mineralization site and mode of calcification in psammoma bodies. They suggested that hydroxyapatite crystals were repeatedly precipitated within the bodies and that this precipitate might gradually aggregate within the bodies, resulting in large psammoma bodies. Collagen fibers surrounding the calcified bodies then accumulated deposits of apatite crystals, forming huge psammoma bodies15). However, Kitagawa et al.13) concluded that the ossification of meningioma is secondary to the metaplasia of the arachnoid cells rather than psammomatous features. Also, Doita et al.6) reported that the calcified psammomatous bodies may not always lead to bone formation. In our case, the histologic examination revealed metaplastic meningioma with bone ossification without psammomatous calcification, the extensive calcium deposits formed crystalline structures rather than the characteristic concentric laminations of psammoma bodies that support this hyposthesis.
Many authors reported that recurrence of calcification of meningioma is rare and variable depending on incomplete removal of the tumor6,7,16). In this case of a extremely rare totally ossified metaplastic meningioma with grossly total resection, the postoperative recurrence and outcomes should be followed up carefully in outpatient clinic to compare with other type of meningioma.
CONCLUSION
Spinal MM is rare and a calcified or ossified meningioma usually have adhesion of the tumor to the surrounding nerve tissue. Adhesions between the tumor and the normal nervous tissue could be meticulously separated and the ossified tumor could be totally resected without complication under Tc-MEPs monitoring. Tc-MEP monitoring is very useful method to guide us to manipulate nervous tissue without compromising function. However, how metaplastic ossification changes occur is not known.
Acknowledgements
This study was supported by research funds from Chosun University 2011.