Factors Related to Outcomes of Subthalamic Deep Brain Stimulation in Parkinson's Disease
Article information
Abstract
Objective
Subthalamic nucleus (STN) deep brain stimulation (DBS) is an effective treatment of choice for patients with advanced idiopathic Parkinson's disease (PD) who have motor complication with medication. The objectives of this study are to analyze long-term follow-up data of STN DBS cases and to identify the factors related to outcomes.
Methods
Fifty-two PD patients who underwent STN DBS were followed-up for more than 3 years. The Unified Parkinsons Disease Rating Scale (UPDRS) and other clinical profiles were assessed preoperatively and during follow-up. A linear regression model was used to analyze whether factors predict the results of STN DBS. We divided the study individuals into subgroups according to several factors and compared subgroups.
Results
Preoperative activity of daily living (ADL) and the magnitude of preoperative levodopa response were shown to predict the improvement in UPDRS part II without medication, and preoperative ADL and levodopa equivalent dose (LED) were shown to predict the improvement in UPDRS part II with medication. In UPDRS part III with medication, the magnitude of preoperative levodopa response was a predicting factor.
Conclusion
The intensity of preoperative levodopa response was a strong factor for motor outcome. And preoperative ADL and LED were strong factors for ADL improvement. More vigorous studies should be conducted to elucidate how levodopa-induced motor complications are ameliorated after STN DBS.
INTRODUCTION
The results of subthalamic nucleus (STN) deep brain stimulation (DBS) in Parkinson's disease (PD) are well-studied2,3,7-9,11,16-18,23,25,27,28,34,36,37). The benefits of STN DBS are not in doubt, and are supported even by long-term follow-up data.
Factors related to surgical outcomes are major concerns in STN DBS. Guehl et al.12) reported that age, intensity of axial symptoms and Unified Parkinson's Disease Rating Scale (UPDRS) II off-medication score before surgery predict dysarthria/hypophonia and postural instability after surgery. Tsai et al.32) reported that older age and non-dopaminergic-responsive axial disability were poor prognostic factors. Welter et al.35) also discussed the clinical predictive factors of STN DBS and insisted that age, disease duration, and severity of levodopa-related motor complications were not predictive, but the decisions to perform surgery on the oldest patients and/or patients with gait and postural disorders who are poorly responsive to levodopa should be weighed carefully. We analyzed the factors related to long-term outcome from our data and discussed these in this paper. In addition, we reviewed the patients who improved dyskinesia without sufficient decrease of levodopa equivalent dose (LED) and discussed such paradoxical improvement.
MATERIALS AND METHODS
We performed STN DBS for total 139 PD between February 2000 and October 2006 in our institute. We reviewed 75 patients with fully available medical record for analysis. Finally, we enrolled 52 patients follow-up for at least 3 years. All the patients were diagnosed with idiopathic PD by an expert movement disorder neurologist and a neurosurgeon. Preoperatively, the UPDRS, Modified Hoehn and Yahr staging (H&Y), Schwab and England Activity of Daily Living (ADL) scale, Beck Depression Inventory (BDI), Mini-Mental Status Examination (MMSE), Clinical Dementia Rating (CDR), and basic neuropsychological tests were assessed. UPDRS part II and III were evaluated with and without medication respectively. Levodopa challenge tests were performed to determine whether patients were suitable for the surgery. That is, UPDRS part III was evaluated by expert movement disorder neurologist after being "off" levodopa medication for a minimum of 12 hours. Then, UPDRS part III evaluation was performed again by same neurologist after levodopa administration (usually 1.5 times the dose). We performed the surgery if the patient showed greater than 33% improvement of UPDRS part III in levodopa challenge test. We excluded the patients with Parkinsonism and any cognitive and psychiatric problems. Surgery was performed in a single day, from bilateral leads implantation to implantable pulse generators insertion. After surgery, the items that had preoperatively evaluated assessed during follow-up.
Statistical analysis
The statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). We first compared the data between the preoperative state and the follow-up state. To compare these differences, the paired t-test was used or parametric variables such as LED and the nonparametric Wilcoxon signed-ranked test was used for nonparametric variables such as UPDRS scores. Second, factors such as age, sex, symptom duration, preoperative ADL, BDI, CDR, MMSE, H&Y, LED and levodopa responsiveness were analyzed by linear regression to determine if they predicted the results of STN DBS. We divided the sample according to sex, age (≤65 years, >65), symptom duration (≤10 years, >10), H&Y (≤3, >3) and average levodopa responsiveness (≤50.02%, >50.02%), ADL (≤60, >70), preoperative LED (≤900 mg/day, >900) and compared the long-term follow-up data of each group. In order to determine the differences between the groups, t-tests and Wilcoxon signed-ranked tests were performed. Test variables were differences in respective scores between preoperative state and final follow-up state in H&Y, ADL, UPDRS score, DBI, MMSE, CDR, and LED. Finally, we compared the previous profiles in the patients that showed an increase in LED and the patients with aggravated results in UPDRS part III. For all analyses, a p<0.05 threshold for significance was chosen.
RESULTS
Demography of the patients
The mean age of the patients at surgery was 57.60±10.58 (range 36-77) years, and the sex ratio (male : female) was 26 : 26. Mean symptom duration was 10.25±4.84 (range 5-22) years, and median follow-up duration was 57.48 (range 37-110) months as shown in Table 1. Preoperative ADL, BDI, MMSE, CDR, daily LED and levodopa response (in UPDRS part III) are shown in Table 2.
Preoperative state versus final follow-up state
There was remarkable improvement between the preoperative state and final follow-up state. The clinical courses over 3 years were shown in Fig. 1. The improvement after surgery maintained through whole follow-up period. Preoperative UPDRS part III without medication was 43.19±15.31 and improved to 29.65±12.44 (p<0.001) finally. LED decreased from 957.16±487.10 to 603.41±359.03 (p<0.001). UPDRS part II with/without medication, UPDRS part IV, H&Y, and ADL scores showed statistically significant improvement (Table 2). UPDRS part I, MMSE and BDI did not show any changes throughout the entire follow-up duration. Only CDR score showed a statistically significant increase in long-term follow-up (p<0.001) (Table 2).
Factors related to long-term results
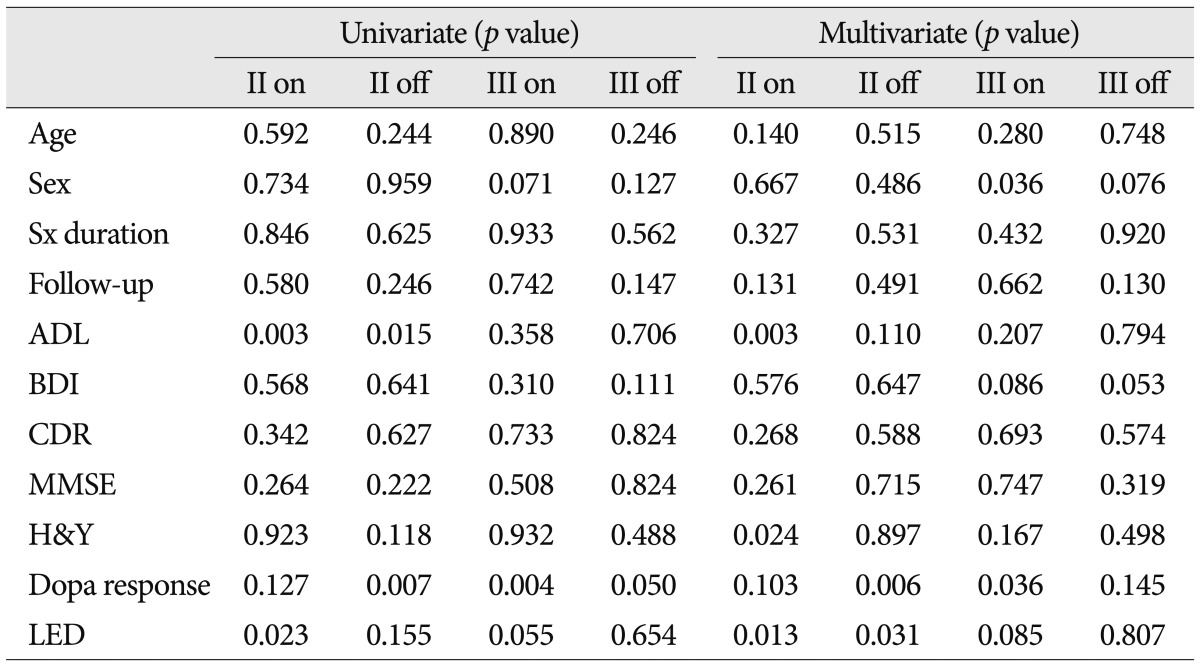
Several factors were analyzed to determine if they predicted improvement in UPDRS parts II and III with or without medication, respectively (Table 3). In the univariate analysis, preoperative ADL (p=0.015, R2=0.112) and the magnitude of preoperative levodopa response (p=0.007, R2=0.136) were shown to predict the improvement in UPDRS part II without medication, and preoperative ADL (p=0.003, R2=0.160) and LED (p=0.023, R2=0.099) were positively correlated with the improvement in UPDRS part II with medication. Proper understanding of the magnitude of preoperative levodopa response (p=0.004, R2=0.156) could lead to consistent improvement in UPDRS part III with medication.
The factors which were significant in univariate analysis proceeded in multivariate analysis. Preoperative ADL (p<0.001) and LED (p=0.013) were related to the improvement in UPDRS part II with medication (R2=0.464). In addition, the magnitude of preoperative levodopa responsiveness (p=0.006) was related to the improvement in UPDRS part II without medication (R2=0.355). The magnitude of preoperative levodopa responsiveness (p=0.036) was related to the improvement in UPDRS part III with medication (R2=0.332).
In order to determine whether factors such as gender, age, H&Y stage, symptom duration and the intensity of preoperative levodopa response influence the results of STN DBS, we divided the study sample into groups and compared them. In the groups divided by sex, male patients showed a greater decrease in UPDRS part II score (only without medication, p=0.039) and part III score (only without medication, p=0.033) than females. In the groups divided by age, young patients showed greater improvement in H&Y stage (p=0.006). In H&Y groups, H&Y 1-3 groups showed greater improvement in UPDRS IV than did the H&Y 4-5 groups (p=0.025). In levodopa responsiveness groups, more improvement in UPDRS part III score (only without medication, p=0.034) was shown as stronger response to levodopa preoperatively (Fig. 2). There were no significant differences in the groups divided by other variances.
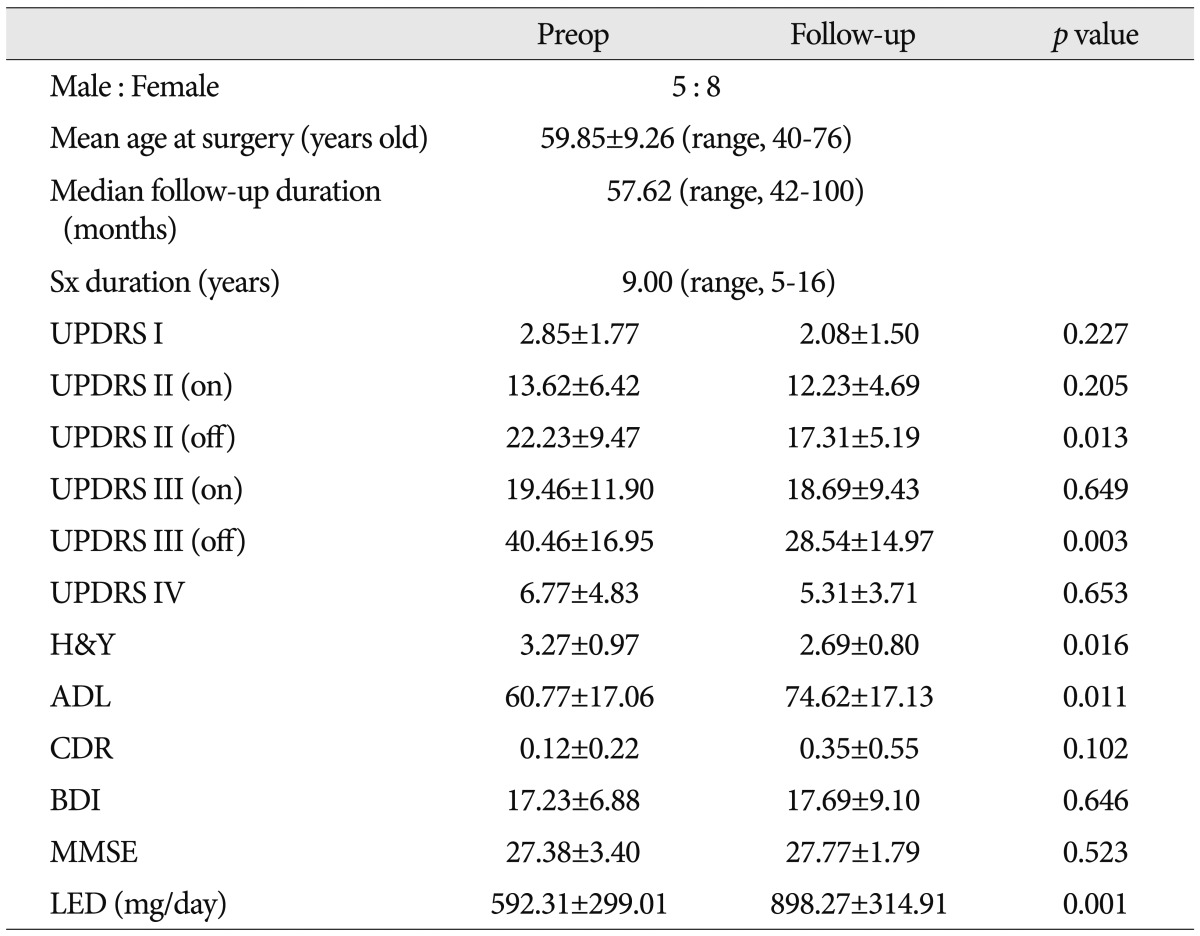
Thirteen patients whose LED increased after long-term follow-up
LED decreased from 957.16±487.10 to 603.41±359.03 (p<0.001) (Table 2). However, there were 13 patients whose LED increased at the final follow-up rather than preoperatively. The sex ratio of theses patient was 5 : 8 (male : female), and their mean age was 59.85± 9.26 years (range, 40-76). UPDRS part II off-medication, III off-medication, H&Y, and ADL in the final follow-up state showed statistically significant improvement compared with preoperative state (Table 4). UPDRS part IV decreased slightly despite the increased medication dosage, although the change was not statistically significant.
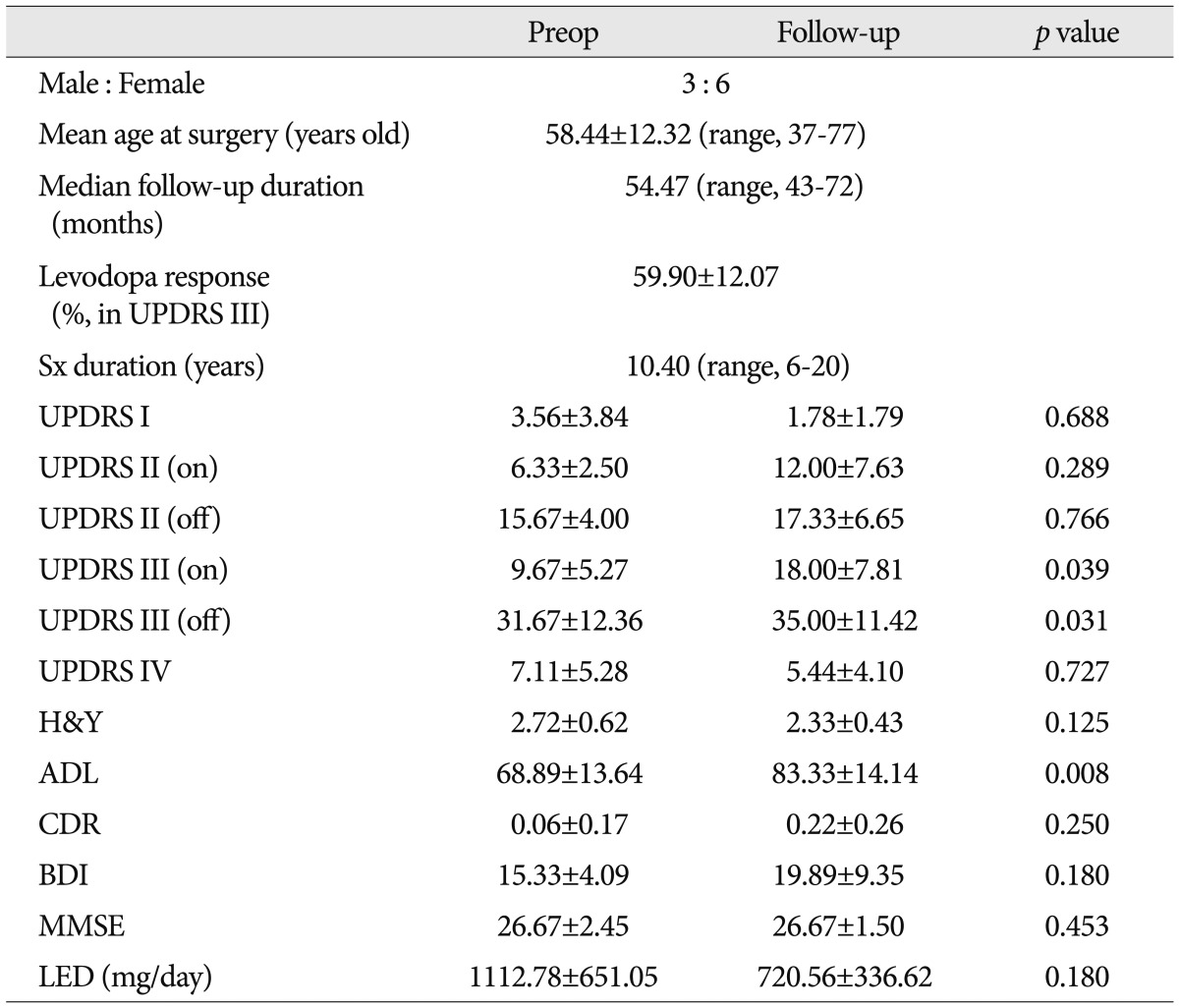
Nine patients whose UPDRS III score deteriorated after long-term follow-up
Overall, the patients showed improvement after STN DBS; however, 9 patients experienced aggravation in UPDRS part III (with/without medication) after long-term follow-up (Table 5). The sex ratio of these patients was 3 : 6 (male : female), and their mean age was 58.44±12.32 years (range, 37-77). No other scores showed statistically significant aggravation, and ADL showed improvement (p=0.008). Among these 9 patients, LED and UPDRS part I score decreased in all except two patients, and UPDRS part IV score decreased in all except three.
Complications
There was no mortality in our series. Complications related to the surgical procedures were hemorrhage and infection. Three patients showed infection, and the leads at the infected sites were removed. Disconnection of leads occurred in one patient, in whom the lead was exchanged. Intracranial hemorrhage occurred in one patient who recovered with some disabilities. The adverse effects related to chronic stimulation were mostly tolerable with parameters controlled in the outpatient department.
DISCUSSION
Long-term follow-up of STN DBS
In general, STN DBS results in improvement in the motor symptoms of PD2,11,36). In our study, UPDRS part II with medication, UPDRS part III with/without medication, UPDRS part IV, LED, H&Y, and ADL showed improvement after long-term follow-up. However, CDR increased in long-term follow-up and is thought to be due to the natural course of PD. Other basic neuropsychological profiles such as UPDRS part I, MMSE and BDI did not show any changes in long-term follow-up. In the literature, it was reported that STN DBS cannot stop the natural progression of PD11,36). Kaiser et al.15) reviewed 38 PD patients who underwent STN DBS and mentioned that their psychosocial profiles transiently improved at 12 months after surgery but returned to baseline at 36 months. Tsai et al.33) reported that neuropsychological effects after STN DBS were closely related to anterior location of the active electrode contact within the ventral STN. Weaver et al.34) reported the effectiveness of DBS compared with best medication treatment in randomized controlled trial. Gervais-Bernard et al.11) reported on 42 prospective PD patients treated with STN DBS and showed that DBS resulted in long-term benefits but did not prevent disease progression. Krack et al.18) reported 5 years of prospective follow-up data in 49 PD patients and concluded that there was marked improvement in motor function without medication and dyskinesia with medication, but worsening of akinesia, speech, postural stability, freezing of gait, and cognitive function that was consistent with the natural history of PD. Wider et al.36) also reported that STN DBS is an effective treatment, but that disease progression occurs in long-term follow-up. Erola et al.9) reported that bilateral STN DBS improved health-related quality of life in 27 PD patients during 12 months. Improvement in motor function and medication-related motor complications must be definitely established, although worsening of some components such as speech, axial symptom, and cognition due to natural course of PD seems to be inevitable.
Effect of gender on STN DBS
The hypothesis that gender could affect the results of STN DBS is mentioned by a few lieteratures1,24). In our series, the magnitude of improvement in UPDRS parts II and III without medication was significantly greater in men than in women. Romito et al.24) mentioned poorer transient outcomes in women patients, similar to our results. Marceglia et al.19) reported differences in local field potential of STN between male PD patients and female PD patients, which may be important for understanding gender-specific features of neurodegenerative disorders. Conversely to our results, Hariz et al.13) concluded that stereotactic surgery for PD patients should be offered more often and earlier in women since female patients had longer duration of disease and higher H&Y scale but experienced greater benefit than male patients in ADL, emotions, and social life. Accollar et al.1) reported that women had greater improvement in ADL. These gender-related differences might be related to sex hormones, hormonal modulation on dopaminergic receptors and gene expression or anatomical and chemical differences, while the subjective answers about ADL might be influenced by social and cultural differences.
Effect of age on STN DBS
It is uncertain whether the results of STN DBS are affected by age. H&Y stage improvement was greater in the young age group than in the elderly group in our study. We did not exclude elderly patients, and elderly patients in our series accounted for 28.8% (15/52) of the total patients. Previous reports have shown that the results of STN DBS are related to age. Tagliati et al.31) and Simuni et al.30) reported that age was not a predictor of STN DBS outcome. Ory-Magne et al.20) reported their results according to patient age and insisted that UPDRS parts II, III, cognitive impairment, and quality of life had no correlation with age, although there was a significant increase in symptomatic cerebral hemorrhage in the elderly group. Derost et al.6) reported that postoperative quality of life improved up to 2 years only in young PD patients. In our series, only H&Y stage improvement was affected by age, and other clinical data were not different between young and elderly patients. We believe that age alone does not determine long-term surgical outcome nor should it be an exclusion criterion of surgical indication.
LED increase after long-term follow-up
STN DBS can decrease LED and levodopa-induced motor complications. Interestingly, the ADL of PD patients showed improvement after surgery even though the LED of those patients increased in our series. H&Y, UPDRS parts II and III (without medication) also improved in those patients. In addition, their UPDRS part IV scores decreased slightly although the decrease was not statistically significant. Motor fluctuations are strongly related to disease duration and levodopa dose and dyskinesia is related to duration of levodopa treatment26). Follett10) mentioned the difference between pallidal stimulation and subthalamic stimulation in regard to treatment of levodopa-induced dyskinesia. They reported that STN DBS mimics the effects of levodopa on parkinsonian motor symptoms and allows reduction of dopaminergic medication, secondarily relieving dyskinesia as medications are reduced or withdrawn postoperatively while pallidal stimulation is aimed directly at decreasing dyskinesia. However, they also observed attenuation of dyskinesia without reduction of medication in some cases. In addition Simonin et al.29) reported that the long-term effects of STN DBS on levodopa-induced motor complications may be explained by the overall stabilization of the basal ganglia network and striatal synaptic changes.
The mechanism of levodopa-induced motor complication is still under investigation at the interface between clinical and basic neuroscience4,26,29). The risk factors for motor complications are known as young onset age, severe disease, longer duration of levodopa therapy, high dose of levodopa and genetic predisposition14,29). Recently, the understanding of the mechanism was expanded to include pre-synaptic abnormalities in dopamine release and clearance, molecular and synaptic adaptations in the striatum, and microvascular changes induced by levodopa4). As our cases showed postoperative clinical improvement although LED increased, the mechanism of STN DBS can affect motor circuits and improve levodopa-induced motor complications is still obscure but is not simply due to reduction of LED.
Deterioration in UPDRS III score after long-term follow-up
The patients who deteriorated in motor function (UPDRS part III) in long-term follow-up did not experience aggravated ADL but rather improvement (p=0.008). These patients showed good preoperative response to levodopa, 59.90%, which was greater than that of the overall population in our series. The cause of deterioration in UPDRS part III was unclear in our study but was assumed to involve the aging process and disease progression. On the other hand, ADL improved in these patients and is closely related to motor fluctuation and drug-induced dyskinesia in advanced PD patients26). STN DBS can effectively reduce the motor complications closely related to ADL. Considering the small number of cases included in this study, the results that 7 of 9 patients improved in LED and 6 of 9 patients improved in UPDRS IV should be carefully considered. These factors such as LED reduction and levodopa-induced complication relief are thought to affect ADL improvement in these patients.
Intracranial electrode position and clinical outcomes after STN DBS
Even though the factor of intracranial electrode position was not included in this study, it would be a very important factor for outcomes after STN DBS. The authors performed surgery under the local anesthesia. Microelectrode recording and intraoperative stimulation test were performed in all cases. Also, we confirmed the position of intracranial electrode in postoperative MRI. The accuracy of electrode position and clinical outcomes were already reported by the authors5). However, a few studies on the electrode position and clinical outcomes were reported. Peak et al.21,22) reported that more accurate electrode positioning in the STN is very important factor for better outcomes after STN DBS.
CONCLUSION
STN DBS showed definite and marked improvement in advanced idiopathic PD. The intensity of preoperative levodopa response was strongly related to motor improvement and ADL, while preoperative ADL and LED were strongly related to ADL improvement after STN DBS. In addition, STN DBS can improve the levodopa-induced motor complications even without reduction of LED. The reduction of levodopa-induced motor complications in advanced disease can improve ADL of PD patients after STN DBS. We do not know exactly how levodopa-induced motor complications are ameliorated after STN DBS, but the mechanism is probably more than secondary LED reduction from improved parkinsonian symptoms and likely to stabilize the motor circuits or to modulate the dopamine synaptic system.
Acknowledgements
This study was financially supported by the grant from the Industrial Source Technology Development Program (no. 10033812) of the Ministry of Knowledge Economy (MKE) of Korea (JW Chang).