The Usefulness of Brain Magnetic Resonance Imaging with Mild Head Injury and the Negative Findings of Brain Computed Tomography
Article information
Abstract
Objective
To investigate the cases of intracranial abnormal brain MRI findings even in the negative brain CT scan after mild head injury.
Methods
During a 2-year period (January 2009-December 2010), we prospectively evaluated both brain CT and brain MRI of 180 patients with mild head injury. Patients were classified into two groups according to presence or absence of abnormal brain MRI finding even in the negative brain CT scan after mild head injury. Two neurosurgeons and one neuroradiologist validated the images from both brain CT scan and brain MRI double blindly.
Results
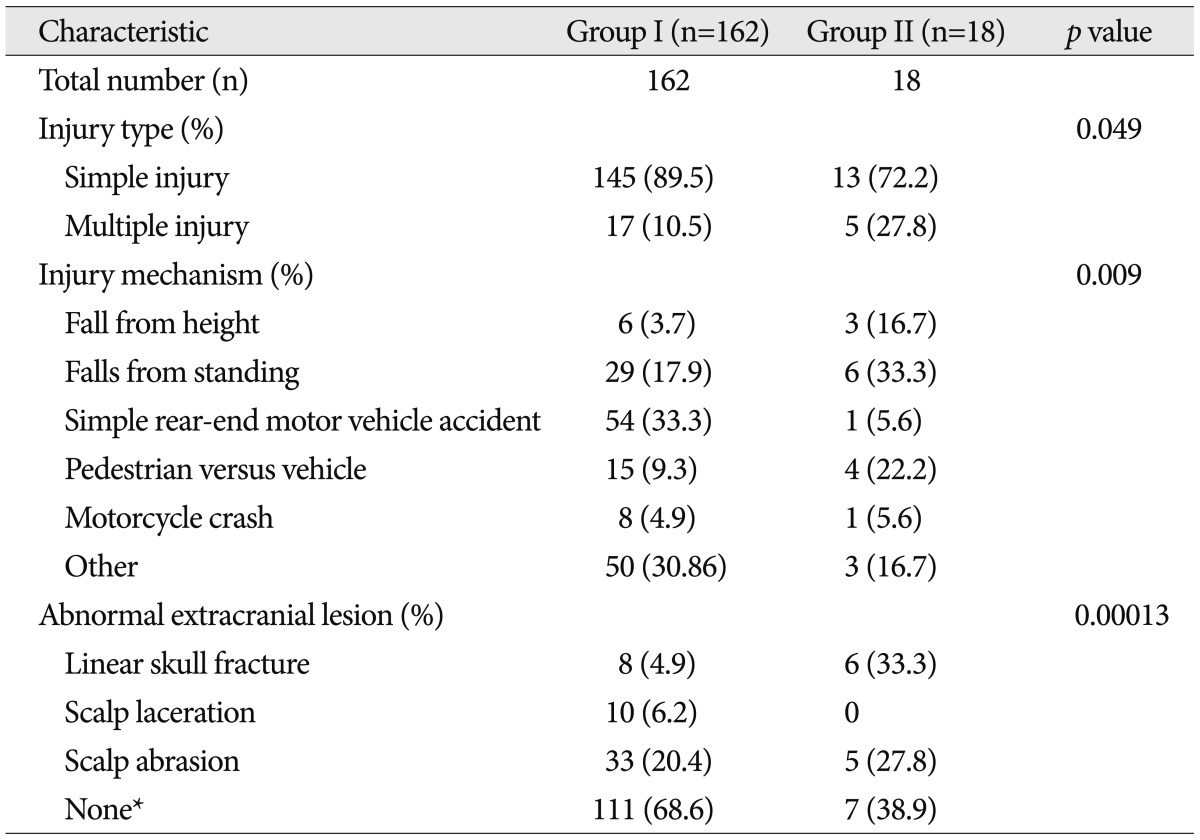
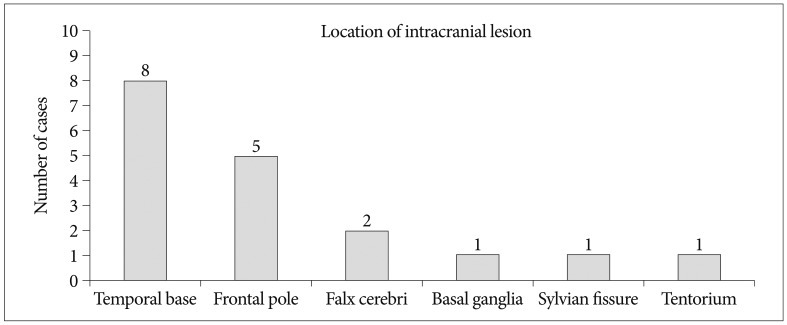
Intracranial injury with negative brain CT scan after mild head injury occurred in 18 patients (10.0%). Headache (51.7%) without neurologic signs was the most common symptom. Locations of intracranial lesions showing abnormal brain MRI were as follows; temporal base (n=8), frontal pole (n=5), falx cerebri (n=2), basal ganglia (n=1), tentorium (n=1), and sylvian fissure (n=1). Intracranial injury was common in patients with a loss of consciousness, symptom duration >2 weeks, or in cases of patients with linear skull fracture (p=0.00013), and also more frequent in multiple associated injury than simple one (35.7%>8.6%) (p=0.105).
Conclusion
Our investigation showed that patients with mild head injury even in the negative brain CT scan had a few cases of intracranial injury. These findings indicate that even though the brain CT does not show abnormal findings, they should be thoroughly watched in further study including brain MRI in cases of multiple injuries and when their complaints are sustained.
INTRODUCTION
Brain CT is still the technique of choice for evaluation of head trauma7,12,13,16,17,22) and it is an important part of the diagnostic armamentarium in a head injury as neuro-cranial imaging2,21,23,24). It is readily obtainable in most medical centers and is the cornerstone for rapid diagnosis5). The availability of CT, its rapidity of scanning, reconstructive ability and compatibility with medical resuscitation devices underlie its use in acute head injury imaging5,10,17,22). Contrast from CT scans, MRI findings typically demonstrate the lesions from the onset of injury, but many facilities cannot perform MRI on an emergent basis. In addition, MRI examination can take up to an hour to perform, and patients may require sedation to minimize motion artifacts. Moreover, national insurance in Korea usually does not cover for MRI if brain CT scan does not show abnormal lesions in head injury patients. Thus, in such cases, doctors have usually skip the components of a further study, such as brain MRI.
Brain CT scan shows commonly normal in mild head trauma settings. However, in a few cases, MRI can show abnormal findings, such as cerebral contusion even in such a case12). It is important to find out whether there is an intracranial injury or not which is not shown in a brain CT due to legal issues, medical insurance, and neuropsychiatric dysfunction.
Few studies have been taken in case of mild head injury to decide whether only a CT scan is sufficient to evaluate it or not and when further study such as MRI is needed. The purpose of this study was to clarify the debate through a study of a large number of patients with negative brain CT after head trauma. This study was designed to determine; 1) the incidence of abnormal brain MRI findings even in the negative brain CT scan, 2) specific cases which MRI is needed in mild head trauma patients with normal intracranial finding of brain CT scan, and 3) which predictable risk factors including patients' characteristics [age, gender, past medical history, associate symptoms, coagulopathy, loss of consciousness, initial Glasgow Coma Scale (GCS) score, symptom duration], injury mechanism, and lesion location are highly related with positive brain MRI finding even if negative intracranial finding of brain CT scan.
MATERIALS AND METHODS
Patients population
From January 1, 2009 to February 28, 2011, we prospectively registered cases of mild head injury having negative brain CT scan, who visited the neurosurgery department of our hospital. During a 2-year period, we prospectively evaluated brain CT and brain MRI of 180 patients with mild head injury.
Mild head injury was defined as GCS score of 13 to 14 or GCS score of 157), with at least one of the following risk factors : history of loss of consciousness, short-term memory deficit, amnesia for the traumatic event, post-traumatic seizure with blunt injury to the head, such as traffic accident, pedestrian trauma, fall down injury, slipped down injury or sports injury11,16,19,24). Persons whose continuous observation were possible during hospitalization and underwent both brain CT and MRI, were included in this study. Patient with a GCS score <13, contraindications for CT, or concurrent intracranial injuries in head CT at presentation were excluded.
Brain CT scan and brain MRI procedures
After the original clinical examinations, all patients underwent standard CT scan of the head according to the judgment of the treating physician. All brain CT scans, 64-MDCT (SOMATOM Sensation, Siemens Medical Solution, München, Germany), were performed within 8 hours of presentation to the hospital and non-contrast axial whole brain CT scans were obtained with 4.8 mm of slices thickness. Also, brain and bone windows were obtained from all patients.
Patients without demographic data on their request forms and poor CT images were excluded. Such poor CT images were laden with motion artifacts interfering with meaningful image evaluations. All the including patients underwent early brain MRI [4.67±2.65 days after injury (mean±SD)] within 7 days after first brain CT scan for confirm whether they have intracranial injuries or not. We performed a MRI protocol on a 3.0-T scanner (Achieva TX, Philips Medical System, Amsterdam, Netherlands) with axial and sagittal T1-weighted sequence, axial T2-weighted sequences, T2 FLAIR, and axial GRE sequence using a 16-in diameter coil.
Saturation recovery images were obtained with a 500-msec repetition time, using averages of four signals, a matrix size of 340×191, a slice thickness of 5 mm, and multislice data acquisition. All studies were interpreted by two neurosurgeons and one neuroradiologist, who had a certificate for added qualification in neuroradiology.
Grouping
According to brain CT and brain MRI findings, two groups were classified as follows; group I (Fig. 1A-E) with negative brain CT and negative brain MRI, group II (Fig. 1F-J) with negative brain CT and positive brain MRI. Patients with intracranial abnormalities of initial brain CT scan after mild head injury or incidental findings of cerebral infarction, brain tumor, or cerebral aneurysm were excluded. 'Negative' CT scan and brain MRI imaging were defined as having no traumatic intracranial lesion, except scalp swelling or simple linear skull fracture. Simple linear skull fracture if it was not combined with intracranial lesion was considered to be 'negative' brain CT scan or brain MRI imaging. Besides, 'positive' brain MRI imaging were considered to show intracranial abnormal finding on brain MRI imaging, such as traumatic intracranial lesion (depressed skull fractures, focal or diffuse contusion, parenchymal hematoma, epidural hematoma, subdural hematoma, subarachnoid hemorrhage).
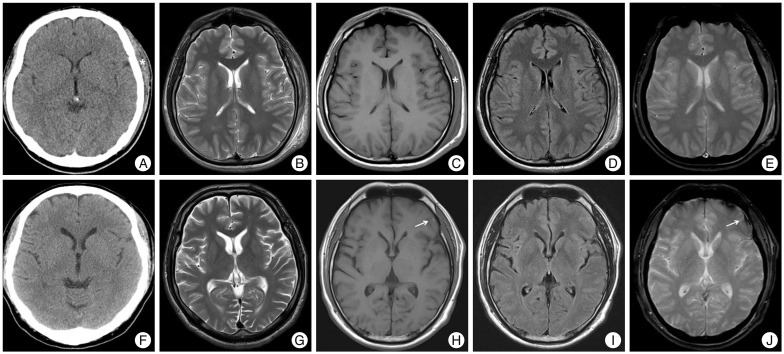
Illustration by magnetic resonance imaging (MRI) compared to computed tomography (CT) in 2 study participants of mild head injury. A 22-year-old male corresponded brain CT (-) and brain MRI (-) group I (A-E) which had no brain CT or MRI parenchymal lesion except extensive scalp swelling (*) of left parieto-occipital area and subgaleal hematoma after mild head injury. A 46-year-old male demonstrated initial brain CT (F) was normal after traffic accident and MRI at post-injury 7 days showed minimal subdural hematoma (arrow) on left temporo-parietal lobe at axial section of T1-weighted image (H) and the hemorrhagic contusion on left frontal lobe (arrow) at axial T2-weighted gradient echo MRI image (J) which corresponded brain CT (-) and brain MRI (+) group II (F-J).
Validation in interpretation of CT scans
Patients were classified by interpretation according to presence or absence of abnormal brain MRI finding. Two neurosurgeons and one neuroradiologist validated the results of interpretation of the images both brain CT scan and brain MRI double blindly. An independent staff who was unaware of the agreement between two sets of readings was analyzed with the use of Cohen's kappa test and the Statistical Package for the Social Sciences version 12.0 software (IBM SPSS Statistics, IBM Corporation, New York, USA).
There was intra-observer agreement between the two interpretations for 20 imaging study as follows : (Kappa : 1.000) for CT, [Kappa : 0.773 (p<0.001), confidence interval (CI) : 0.527-0.907] for MRI. To determine the reproducibility of the CT scan and MRI data, 20 patients were reviewed and examined by a second physician at the time of the initial evaluation. There was inter-observer agreement between the two sets of evaluations for 20 patients as follows : (Kappa : 1.000) for CT, [Kappa : 0.773 (p<0.001), CI : 0.527-0.907] for MRI.
Data collection
Data from the electric charts were tabulated into patient's characteristics and injury characteristics categories. Two trained data collectors performed data entry. The principal investigators reviewed total medical records in order to determine reliability and validity of the data collection method.
These data included in age, gender, past medical history, coagulopathy, main symptom, loss of consciousness, initial GCS score, symptom duration, injury type, mechanism of injury, and trauma type. According to the injury type, simple injury was defined as head trauma only, and multiple injury was defined as head trauma with other tissue injury. Coagulopathy was defined as history of bleeding or clotting disorder or current treatment with aspirin, clopidogrel or warfarin17,18). Low initial GCS score was defined as GCS score of 13 to 14.
In Table 1, the chi-square test in order to validate the significance of gender, main symptom, the loss of consciousness, and symptom duration. The Fisher's exact test was used in order to validate the significance of past medical history, coagulopathy, and initial GCS score. Fisher's exact test for injury type and chi-square test for injury mechanism, and abnormal extracranial lesion were used for significance testing. Statistical significance was defined as p-values <0.05. Statistical data were analyzed using SPSS 12.0 statistical package.
Case illustration
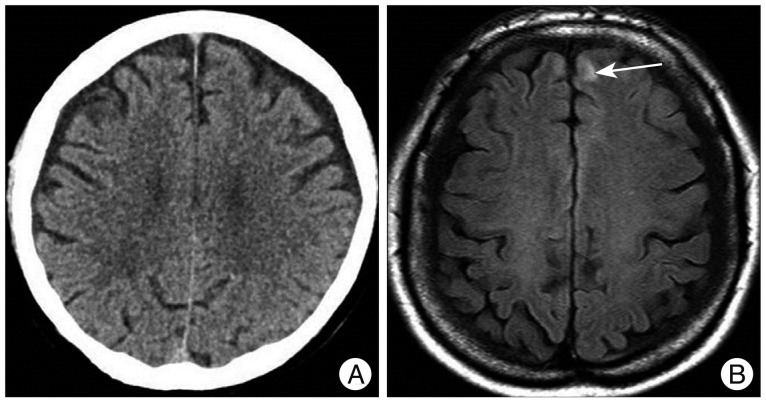
A 53-year-old woman visited our hospital because of headache after pedestrian traffic accident. She had past medical history of hypertension and not taken any other medications. At the time of admission, GCS score was 14 points, and she had loss of consciousness. She showed normal level of coagulation factor on routine hematology examination. Except for scalp laceration, external wound of the other head parts was not observed. Initial brain CT scan which was taken at other institution did not show abnormal lesion, except scalp swelling. She had continuous headache and dizziness for 3 days. Thus, we performed brain MRI which showed hemorrhagic contusion at left frontal lobe (Fig. 2). After we managed with medication and closed observation, she was discharged without any neurologic deficit 1 week later.
RESULTS
Incidence of negative CT scan with positive MRI finding
Table 1 shows patients' characteristics. Eighteen patients (10.0%) of 180 after mild head injury had intracranial injury, even though they had not intracranial abnormal brain CT finding. All patients did not require surgical intervention and management in intensive care unit.
Age, gender, past medical history, and coagulopathy
The mean age of the 180 patients was 48.9 years (range, 13 to 88), and 51% were male. The mean ages for group I and II were 48.2 years and 54.6 years respectively. In group I, female (n=83) was more common than male (n=79) and male (n=12) was more common than female (n=6) in group II. However, there was no statistical significance between the two groups in the age (p=0.083) and gender (p=0.233) (Table 1).
A total of 71 patients had past medical history, including 42 patients (23.3%) with hypertension, 14 patients (7.8%) with diabetes, 7 patients (3.9%) with liver disease, and 8 patients (4.4%) with others. A total of 26 patients had coagulopathy. Neither of past medical history nor coagulopathy was statistically significant between the two groups.
Symptoms of mild head trauma
Persistent headache (51.7%) without neurologic signs was the most common symptom. Thirty patients (16.7%) had dizziness, 13 patients (7.2%) had blurred vision, 8 patients (4.4%) had insomnia, and 36 patients had others. Symptoms between two groups were not statistically significant (p=0.560). Fifty patients had a loss of consciousness. Ten patients (55.6%) of the group II patients and 40 patients (24.7%) of the group I patients had loss of consciousness. Compared with group I, group II has a higher proportion than group I with the loss of consciousness, which is statistically significant (p=0.006).
Fifteen of the patients had low initial GCS score. Group II showed higher percentage of low initial GCS score than those of group I (28% vs. 6%). Patients presenting initial low GCS score was statistically significant to have intracranial lesions (p=0.009).
Most of them, symptoms of 102 cases sustained less than 2 weeks (56.7%). As for symptoms lasting more than two weeks of the entire inter-group analysis, there were 63 cases (38.9%) of group I compared with that (83.8%) of group II which was significantly different (p=0.0003).
Injury type
Table 2 shows the comparison of injury characteristics by groups. Most of them were linear skull fracture (n=14). Other injury types, except 115 cases without external damage, were as follows : scalp laceration (n=10) and scalp abrasion (n=38). Cases of presenting skull fracture were more frequent in group II (p=0.00013). The most common cause of head injury was 55 cases (30.6%) of simple rear-end motor vehicle crashes. Injury mechanisms were standing fall [35 (19.4%) cases], pedestrian versus vehicle [19 (10.6%) cases], motorcycle crash, and fall from height [9 (5.0%) cases] were in row. Large impact trauma, such as fall from height or pedestrian versus vehicle, was relatively frequent than simple rear-end motor vehicle crash, which refers less impact trauma such as in group II. Simple injury was more common than multiple injury (158 cases vs. 22 cases), but multiple injury in group II was significantly frequent than group I cases (27.8% vs. 10.5%) (p=0.049).
Analysis of 18 patients in group II
We found positive findings on brain MRI in 18 patients. These findings included subdural hematoma [5 (27.8%) cases], single focal contusion [5 (27.8%) cases], diffuse cerebral contusion [5 (27.8%) cases], subarachnoid hemorrhage [2 (11.1%) cases], and epidural hematoma [1 (5.6%) case] (Table 3). The most common location of abnormal brain contusion after mild head injury was a temporal base (n=8), and second location was a frontal pole (n=5). In addition, our study showed the other location as follows : falx cerebri (n=2), basal ganglia (n=1), sylvian fissure (n=1), and tentorium (n=1) (Fig. 3).
DISCUSSION
Approximately two thirds of patients with head trauma in the United States are classified as having mild head injury5,11). Mild head injury include patients with scores of 13 to 15 on the GCS, indicating little or no impairment in the consciousness4,5,12,13,15-17,19,20,23). Mild head injury may be unstable and uncooperative2,4,19,23). Thus, it is important to exclude whether there is brain contusion or not in such a case. In early 1990s, several retrospective studies of patients with minor head injury reported substantial proportions with intracranial lesions on CT (17 to 20%)5). Head CT images obtained immediately after the traumatic event often show no evidence of brain swelling or edema, but practically normal3,6). Approximately less than 10% of patients with mild head injury in the United States had positive findings on a brain CT scanning, implying that greater than 90% had normal CT findings5,15). In another study of patients with a score of 15 on the GCS, the rate of intracranial lesions on brain CT was similar (6 to 9%)2).
CT is considered as the first choice in the assessment of the patients with acute head injury15,22). A CT scan is probably recommended for all patients because one in five will have an acute lesion detectable by the scan with head injury. It can be performed quickly; newer CT scanners can complete a scan within 5 minutes. CT scan findings help identify abnormalities that may need acute intervention and can be performed in the presence of life support equipment. Thus, it is easily accessible in most hospitals and a good screening tool to triage mild head injury so as to ascertain who should be safely discharged home or admitted22). CT is the preferred tool for skull lesions and the sensitivity of CT for significantly higher than MRI for evaluation of fracture. In addition, advantageously poor quality CT images, due to motion blurring, are easily repeated.
Compared many advantage of CT scan, it has several weak points in mild head injury. The true volume of neuronal damage in the contused brain tissue can be underestimated. The detection of superficial contusions using CT scans is hampered by artifacts from adjacent bone. Imaging findings in brain contusions tend to vary because of the stages of evolution common to these lesions. Initially, CT findings can be normal or minimally abnormal because the partial volumes between the dense microhemorrhages and the hypodense edema can render contusions isoattenuating relative of the surrounding brain8).
When patient, whose initial brain CT was normal, complains of continuous symptoms after discharge, brain MRI may show an abnormal intracranial pathology. This is reason why MRI is the choice for full assessment of brain lesions after head injury. MRI is more sensitive and accurate than CT for detecting contusions because of its multiplanar capability and greater sensitivity for edema8,9). MRI has clear advantage over CT in the evaluation of lesions seen in minor traumatic brain injury like nonhemorrhagic cortical contusions, and follow-up parenchymal changes8). Also, the sensitivity of MRI is significantly higher than CT in detecting diffuse axonal injury, brain stem lesions, non-hemorrhagic contusion or subacute subdural bleed and sinus invasion8,22). Streak and beam hardening artifacts particularly degrade the imaging of lesions close to the cerebral convexities22). On these regards, MRI with superior soft tissue contrast and multi-planarity is more sensitive for chronic traumatic head injury, subtle abnormality and has strong correlations with long term neuropsychological outcome22).
MRI findings typically demonstrate the lesions from the onset of injury, but many facilities cannot perform MRI on an emergent basis. Estimation of lesion volume based on MRI was frequently greater than with that of CT. In addition, MRI examination can take up to an hour to perform, and patients may require sedation to minimize motion artifacts. Longer scanning time, poor sensitivity to skull fractures and SAH as well as the inability to monitor patients in the MRI fields are its drawbacks22). But, in mild head injury, false negative brain CT scan possibly include insensitive to concussion, diffuse axonal injury, early cerebral edema and has poor resolution in imaging of posterior cranial fossa22). MRI outperforms CT in visualization of small intraparenchymal abnormalities as small contusions and small foci of traumatic axonal injury14). If CT cannot demonstrate pathology, MRI is warranted to adequately be explained to account for clinical state.
MRI have provided tremendous insight into subtle structural abnormalities that are unappreciated by CT. Yuh et al.25) reported that 27% of mild traumatic brain injury patients with normal admission head CT had abnormal early brain MRI which was performed 12±39 days after injury. They had shown that a subset of mild traumatic brain injury patients have significant alterations in neuropsychiatric functioning within weeks to months of injury, and approximately 15% have measurable deficits at 1 year. Our study has shown that 10 percent of patients after mild head injury had positive findings on brain CT scanning and had no required neurosurgical intervention15).
As MRI becomes more widely available, it may have a greater role in the evaluation of patients with mild head injury. Diffusion tensor imaging is more sensitive to white matter injury than conventional MRI and CT. Recently, the use of diffusion tensor imaging of mild traumatic brain injury14) is especially important in cases where the patient experiences chronic postconcussive symptoms despite unrevealing conventional imaging. The key point is cost-benefit ratio, which is maximizing price efficacy. MRI cannot always be performed in all patients with mild head injury due to high prices. If so, when would MRI be really indicated in the patients with negative brain CT scan in mild head injury? Its investigation is the core of this study. The use of clinical findings as predictors of intracranial lesions in patients with mild head injury has been evaluated in several studies1,4). Our study had meaningful understandable results about two views, such as 1) patients characteristics and 2) injury mechanism to find out predictable risk factors of intracranial pathology in group II.
Head trauma at the age of 60, coagulopathy, history of neurosurgical procedure or epilepsy, and drug or alcohol consumption were widely known risk factors for intracranial pathology on a brain CT scan after mild head injury1,11,16,21,24). But in our survey, they were not significant. In our study, group II of higher proportion of patients with loss of consciousness, initial low GCS score, and symptoms duration showed statistically significant risk factors. Thus, careful investigation on these factors especially in mild head trauma patients should be executed.
When head trauma patients admit to the hospital, investigator often overlooks the information about the injury mechanism. Our study provides that this information is important to predict intracranial pathology in patients with negative brain CT scan after mild head injury. Such as falls from height, falls from standing, pedestrian traffic accident, rather than simple rear-end motor vehicle accident, demonstrated more significantly common in group II patients. In the trauma type, group II showed having a high percentage of linear skull fracture rather than external lesions such as scalp laceration or abrasion. These results suggest that they may cause to exert more direct blow to make an occurrence of the intracranial pathology in patients with negative brain CT scan after mild head injury than simple accident.
Multiple injury has a higher percentage of group II than simple injury. In addition, long duration of symptoms (>2 weeks) was significantly higher percentage in group II than group I. Accordingly, the authors recommend that doctors should be alert in cases of multiple injury and complaining of more than 2 weeks. As above results, even the negative brain CT, brain MRI should be recommended to perform especially in limited cases of accompanying those risk factors.
Although our study consisted of 180 consecutive trauma patients, some limitations exist. During time interval between brain CT and brain MRI, if trauma progresses, new intracranial pathology which was absent in the initial brain CT can be visible in brain MRI at a later point in time. Although this hypothesis occur probably quite low because this study is confined in mild head injury patients with negative initial brain CT scan, this is a weak point of our study, and further study is needed in the future.
CONCLUSION
Our study shows that a few patients (10%) with mild head injury, even in negative brain CT scan have intracranial pathology on brain MRI. It was more commonly associated with multiple injury than simple one and when the patients complain sustained symptoms more than 2 weeks, with LOC, and with initial low GCS score after mild head injury. Thus, physicians including neurosurgeons should be alert and do not hesitate to perform brain MRI to carefully watch the regions of temporal base, frontal pole, or falx cerebri in cases of mild head injury accompanying these risk factors.